Abstract
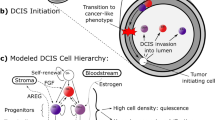
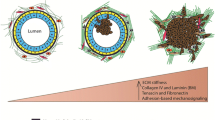
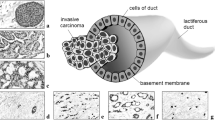
Ductal carcinoma in situ (DCIS) is an early stage noninvasive breast cancer that originates in the epithelial lining of the milk ducts, but it can evolve into comedo DCIS and ultimately, into the most common type of breast cancer, invasive ductal carcinoma. Understanding the progression and how to effectively intervene in it presents a major scientific challenge. The extracellular matrix (ECM) surrounding a duct contains several types of cells and several types of growth factors that are known to individually affect tumor growth, but at present the complex biochemical and mechanical interactions of these stromal cells and growth factors with tumor cells is poorly understood. Here we develop a mathematical model that incorporates the cross-talk between stromal and tumor cells, which can predict how perturbations of the local biochemical and mechanical state influence tumor evolution. We focus on the EGF and TGF-β signaling pathways and show how up- or down-regulation of components in these pathways affects cell growth and proliferation. We then study a hybrid model for the interaction of cells with the tumor microenvironment (TME), in which epithelial cells (ECs) are modeled individually while the ECM is treated as a continuum, and show how these interactions affect the early development of tumors. Finally, we incorporate breakdown of the epithelium into the model and predict the early stages of tumor invasion into the stroma. Our results shed light on the interactions between growth factors, mechanical properties of the ECM, and feedback signaling loops between stromal and tumor cells, and suggest how epigenetic changes in transformed cells affect tumor progression.



























Similar content being viewed by others
References
Abdollah, S., Macias-Silva, M., Tsukazaki, T., Hayashi, H., Attisano, L., & Wrana, J. L. (1997). TbetaRI phosphorylation of smad2 on ser465 and ser467 is required for smad2-smad4 complex formation and signaling. J. Biol. Chem., 272(44), 27678–27685.
Adams, S., Miller, G. T., Jesson, M. I., Watanabe, T., Jones, B., & Wallner, B. P. (2004). PT-100, a small molecule dipeptidyl peptidase inhibitor, has potent antitumor effects and augments antibody-mediated cytotoxicity via a novel immune mechanism. Cancer Res., 64(15), 5471–5480.
Alessi, D. R., Cuenda, A., Cohen, P., Dudley, D. T., & Saltiel, A. R. (1995). PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase in vitro and in vivo. J. Biol. Chem., 270(46), 27489–27494.
Alexander, S., & Friedl, P. (2012). Cancer invasion and resistance: interconnected processes of disease progression and therapy failure. Trends Mol. Med., 18(1), 13–26.
Almholt, K., Green, K., Juncker-Jensen, A., Nielsen, B., Lund, L., & Romer, J. (2007). Extracellular proteolysis in transgenic mouse models of breast cancer. J. Mammary Gland Biol. Neoplasia, 12(1), 83–97.
Annabi, B., Bouzeghrane, M., Currie, J. C., Hawkins, R., Dulude, H., Daigneault, L., Ruiz, M., Wisniewski, J., Garde, S., Rabbani, S. A., Panchal, C., Wu, J. J., & Beliveau, R. (2005). A PSP94-derived peptide PCK3145 inhibits MMP-9 secretion and triggers CD44 cell surface shedding: implication in tumor metastasis. Clin. Exp. Metastasis, 22(5), 429–439.
Basbaum, C. B., & Werb, Z. (1996). Focalized proteolysis: a spatial and temporal regulation of extracellular matrix degradation at the cell surface. Curr. Opin. Cell Biol., 8, 731–738.
Beacham, D. A., & Cukierman, E. (2005). Stromagenesis: the changing face of fibroblastic microenvironments during tumor progression. In Seminars in cancer biology. (Vol. 15, pp. 329–341). Amsterdam: Elsevier.
Brown, D. R. (1999). Dependence of neurones on astrocytes in a coculture system renders neurones sensitive to transforming growth factor beta1-induced glutamate toxicity. J. Neurochem., 72(3), 943–953.
Burrai, G. P., Mohammed, S. I., Miller, M. A., Marras, V., Pirino, S., Addis, M. F., Uzzau, S., & Antuofermo, E. (2010). Spontaneous feline mammary intraepithelial lesions as a model for human estrogen receptor- and progesterone receptor-negative breast lesions. BMC Cancer, 10, 156.
Chen, S. T., Pan, T. L., Juan, H. F., Chen, T. Y., Lin, Y. S., & Huang, C. M. (2008). Breast tumor microenvironment: proteomics highlights the treatments targeting secretome. J. Proteome Res., 7, 1379–1387.
Cheng, J. D., & Weiner, L. M. (2003). Tumors and their microenvironments: tilling the soil commentary re: A.M. Scott et al., A phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin. Cancer Res., 9(5), 1590–1595.
Cheng, G., Tse, J., Jain, R. K., & Minn, L. L. (2009). Micro-environmental mechanical stress controls tumor spheroid size and morphology by suppressing proliferation and inducing aopotosis in cancer cells. PLoS ONE, 4, e4632.
Chivukula, M., Bhargava, R., Tseng, G., & Dabbs, D. J. (2009). Clinicopathologic implications of flat epithelial atypia in core needle biopsy specimens of the breast. Am. J. Clin. Pathol., 131, 802–808.
Chung, S. W., Miles, F. L., Sikes, R. A., Cooper, C. R., Farach-Carson, M. C., & Ogunnaike, B. A. (2009). Quantitative modeling and analysis of the transforming growth factor beta signaling pathway. Biophys. J., 96(5), 1733–1750.
Dallon, J. C., & Othmer, H. G. (1997). A discrete cell model with adaptive signalling for aggregation of Dictyostelium discoideum. Philos. Trans. R. Soc. Lond. B, 352, 391–417.
Dallon, J. C., & Othmer, H. G. (2004). How cellular movement determines the collective force generated by the Dictyostelium discoideum slug. J. Theor. Biol., 231, 203–222.
Danielsen, T., & Rofstad, E. K. (1998). VEGF, bFGF and EGF in the angiogenesis of human melanoma xenografts. Int. J. Cancer, 76(6), 836–841.
Davis, R. J. (1993). The mitogen-activated protein kinase signal transduction pathway. J. Biol. Chem., 268(20), 14553–14556.
Friedl, P., & Alexander, S. (2011). Cancer invasion and the microenvironment: plasticity and reciprocity. Cell, 147(5), 992–1009.
Geho, D. H., Bandle, R. W., Clair, T., & Liotta, L. A. (2005). Physiological mechanisms of tumor-cell invasion and migration. Physiology (Bethesda), 20, 194–200.
Hanamura, N., Yoshida, T., Matsumoto, E., Kawarada, Y., & Sakakura, T. (1997). Expression of fibronectin and tenascin-c mrna by myofibroblasts, vascular cells and epithelial cells in human colon adenomas and carcinomas. Int. J. Cancer, 73(1), 10–15.
Helmlinger, G., Netti, P. A., Lichtenbeld, H. C., Melder, R. J., & Jain, R. K. (1997). Solid stress inhibits the growth of multicellular tumor spheroids. Nat. Biotechnol., 15(8), 778–783.
Hendriks, B. S., Orr, G., Wells, A., Wiley, H. S., & Lauffenburger, D. A. (2005). Parsing ERK activation reveals quantitatively equivalent contributions from epidermal growth factor receptor and HER2 in human mammary epithelial cells. J. Biol. Chem., 280(7), 6157–6169.
Hillen, T. (2006). M5 mesoscopic and macroscopic models for mesenchymal motion. J. Math. Biol., 53, 585–616.
Hillen, T., Hinow, P., & Wang, Z. A. (2010). Mathematical analysis of a kinetic model for cell movement in network tissues. Discrete Contin. Dyn. Syst., Ser. B, 14(3), 1055–1080.
Hinshelwood, R. A., Huschtscha, L. I., Melki, J., Stirzaker, C., Abdipranoto, A., Vissel, B., Ravasi, T., Wells, C. A., Hume, D. A., Reddel, R. R., & Clark, S. J. (2007). Concordant epigenetic silencing of transforming growth factor-signaling pathway genes occurs early in breast carcinogenesis. Cancer Res., 67(24), 11517.
Ilina, O., Bakker, G. J., Vasaturo, A., Hofman, R. M., & Friedl, P. (2011). Two-photon laser-generated microtracks in 3D collagen lattices: principles of MMP-dependent and -independent collective cancer cell invasion. Phys Biol., 8(1), 015010.
Kaufman, L. J., Brangwynne, C. P., Kasza, K. E., Filippidi, E., Gordon, V. D., Deisboeck, T. S., & Weitz, D. A. (2005). Glioma expansion in collagen I matrices: analyzing collagen concentration-dependent growth and motility patterns. Biophys. J. BioFAST, 89, 635–650.
Kim, Y., & Friedman, A. (2010). Interaction of tumor with its microenvironment: a mathematical model. Bull. Math. Biol., 72(5), 1029–1068.
Kim, Y., Stolarska, M., & Othmer, H. G. (2007). A hybrid model for tumor spheroid growth in vitro I: theoretical development and early results. Math. Models Methods Appl. Sci., 17, 1773–1798.
Kim, Y., Lawler, S., Nowicki, M., Chiocca, E., & Friedman, A. (2009). A mathematical model of brain tumor: pattern formation of glioma cells outside the tumor spheroid core. J. Theor. Biol., 260, 359–371.
Kim, Y., Stolarska, M. A., & Othmer, H. G. (2011). The role of the microenvironment in tumor growth and invasion. Prog. Biophys. Mol. Biol., 106, 353–379.
Kloft, C., Graefe, E., Tanswell, P., Scott, A., Hofheinz, R., Amelsberg, A., & Karlsson, M. (2004). Population pharmacokinetics of sibrotuzumab, a novel therapeutic monoclonal antibody, in cancer patients. Invest. New Drugs, 22(1), 39–52.
Koka, S., Vance, J. B., & Maze, G. I. (1995). Bone growth factors: potential for use as an osseointegration enhancement technique (OET). J. West. Soc. Periodontol., Periodontal Abstr., 43(3), 97–104.
Kretzschmar, M., Doody, J., & Massagué, J. (1997). Opposing BMP and EGF signalling pathways converge on the TGFb family mediator smad1. Nature, 389(6651), 618–622.
Kretzschmar, M., Doody, J., Timokhina, I., & Massagué, J. (1999). A mechanism of repression of TGFbeta/ smad signaling by oncogenic ras. Genes Dev., 13(7), 804–816.
Krouskop, T. A., Wheeler, T. M., Kallel, F., Garra, B. S., & Hall, T. (1998). Elastic moduli of breast and prostate tissues under compression. Ultrason. Imag., 20(4), 260–274.
Kudlow, J. E., Cheung, C. Y., & Bjorge, J. D. (1986). Epidermal growth factor stimulates the synthesis of its own receptor in a human breast cancer cell line. J. Biol. Chem., 261(9), 4134–4138.
Kunz-Schughart, L. A., Wenninger, S., Neumeier, T., Seidl, P., & Knuechel, R. (2003). Three-dimensional tissue structure affects sensitivity of fibroblasts to TGF-beta 1. Am. J. Physiol., Cell Physiol., 284(1), C209–C219.
Liu, X., Sun, Y., Constantinescu, S. N., Karam, E., Weinberg, R. A., & Lodish, H. F. (1997). Transforming growth factor beta-induced phosphorylation of smad3 is required for growth inhibition and transcriptional induction in epithelial cells. Proc. Natl. Acad. Sci. USA, 94(20), 10669–10674.
Mantzaris, N., Webb, S., & Othmer, H. G. (2004). Mathematical modeling of tumor-induced angiogenesis. J. Math. Biol., 49, 111–187.
Marino, S., Hogue, I. B., Ray, C. J., & Kirschner, D. E. (2008). A methodology for performing global uncertainty and sensitivity analysis in systems biology. J. Theor. Biol., 254(1), 178–196.
Marshall, C. J. (1995). Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell, 80(2), 179–185.
Massagué, J. (1998). TGF-beta signal transduction. Annu. Rev. Biochem., 67(1), 753.
Massagué, J. (2008). TGF [beta] in cancer. Cell, 134(2), 215–230.
Mercapide, J., Cicco, R., Castresana, J. S., & Klein-Szanto, A. J. (2003). Stromelysin-1/matrix metalloproteinase-3 (MMP-3) expression accounts for invasive properties of human astrocytoma cell lines. Int. J. Cancer, 106(5), 676–682.
Palsson, E., & Othmer, H. G. (2000). A model for individual and collective cell movement in dictyostelium discoideum. Proc. Natl. Acad. Sci., 97, 11448–11453.
Paszek, M. J., & Weaver, V. M. (2004). The tension mounts: mechanics meets morphogenesis and malignancy. J. Mammary Gland Biol. Neoplasia, 9(4), 325–342. Review.
Preziosi, L., & Vitale, G. (2011). A multiphase model of tumour and tissue growth including cell adhesion and plastic re-organisation. Math. Models Methods Appl. Sci., 21, 1901–1932.
Provenzano, P., Inman, D., Eliceiri, K., & Keely, P. (2009). Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK–ERK linkage. Oncogene, 28, 4326–4343.
Renkawitz, J., & Sixt, M. (2010). Mechanisms of force generation and force transmission during interstitial leukocyte migration. EMBO Rep., 11(10), 744–750.
Renkawitz, J., Schumann, K., Weber, M., Lämmermann, T., Pflicke, H., Piel, M., Polleux, J., Spatz, J. P., & Sixt, M. (2009). Adaptive force transmission in amoeboid cell migration. Nat. Cell Biol., 11(12), 1438–1443.
Saffarian, S., Collier, I. E., Marmer, B. L., Elson, E. L., & Goldberg, G. (2004). Interstitial collagenase is a Brownian ratchet driven by proteolysis of collagen. Science, 306(5693), 108–111.
Samoszuk, M., Tan, J., & Chorn, G. (2005). Clonogenic growth of human breast cancer cells co-cultured in direct contact with serum-activated fibroblasts. Breast Cancer Res., 7, R274–R283.
Santos, S. D. M., Verveer, P. J., & Bastiaens, P. I. H. (2007). Growth factor-induced MAPK network topology shapes Erk response determining PC-12 cell fate. Nat. Cell Biol., 9(3), 324–330.
Sappino, A. P., Skalli, O., Jackson, B., Schurch, W., & Gabbiani, G. (1988). Smooth-muscle differentiation in stromal cells of malignant and non-malignant breast tissues. Int. J. Cancer, 41(5), 707–712.
Schmierer, B., Tournier, A. L., Bates, P. A., & Hill, C. S. (2008). Mathematical modeling identifies smad nucleocytoplasmic shuttling as a dynamic signal-interpreting system. Proc. Natl. Acad. Sci. USA, 105(18), 6608–6613.
Sherratt, J. A., & Murray, J. D. (1990). Models of epidermal wound healing. Proc. R. Soc. Lond. B, 241, 29–36.
Shi, Y., & Massagué, J. (2003). Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell, 113(6), 685–700.
Souchelnytskyi, S., Tamaki, K., Engstrom, U., Wernstedt, C., ten Dijke, P., & Heldin, C. H. (1997). Phosphorylation of ser465 and ser467 in the C terminus of smad2 mediates interaction with smad4 and is required for transforming growth factor-beta signaling. J. Biol. Chem., 272(44), 28107–28115.
Stein, A. M., Demuth, T., Mobley, D., Berens, M., & Sander, L. M. (2007). A mathematical model of glioblastoma tumor spheroid invasion in a three-dimensional in vitro experiment. Biophys. J., 92(1), 356–365.
Stein, A. M., Vader, D. A., Weitz, D. A., & Sander, L. M. (2011). The micromechanics of three-dimensional collagen-I gels. Complexity, 16(4), 22–28.
Thorne, R. G., Hrabetova, S., & Nicholson, C. (2004). Diffusion of epidermal growth factor in rat brain extracellular space measured by integrative optical imaging. J. Neurophysiol., 92(6), 3471–3481.
Tlsty, T. D. (2001). Stromal cells can contribute oncogenic signals. Semin. Cancer Biol., 11(2), 97–104.
van den Hooff, A. (1988). Stromal involvement in malignant growth. Adv. Cancer Res., 50, 159–196.
Wakefield, L. M., Smith, D. M., Masui, T., Harris, C. C., & Sporn, M. B. (1987). Distribution and modulation of the cellular receptor for transforming growth factor-beta. J. Cell Biol., 105(2), 965–975.
Wells, C. A., & El-Ayat, G. A. (2007). Non-operative breast pathology: apocrine lesions. J. Clin. Pathol., 60, 1313–1320.
Wolf, K., Wu, Y. I., Liu, Y., Geiger, J., Tam, E., Overall, C., Stack, M. S., & Friedl, P. (2007). Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat. Cell Biol., 9(8), 893–904.
Woodcock, E. A., Land, S. L., & Andrews, R. K. (1993). A low affinity, low molecular weight endothelin-A receptor present in neonatal rat heart. Clin. Exp. Pharmacol. Physiol., 20(5), 331–334.
Zi, Z., Feng, Z., Chapnick, D. A., Dahl, M., Deng, D., Klipp, E., Moustakas, A., & Liu, X. (2011). Quantitative analysis of transient and sustained transforming growth factor-beta signaling dynamics. Mol. Syst. Biol., 7(492), 1–12.
Acknowledgements
Y.J.K. was supported by a Faculty research grant from the University of Michigan–Dearborn and the faculty research fund of Konkuk University in 2012. H.G.O. was supported in part by NSF grant DMS-0517884, and NIH grant GM-29123.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, Y., Othmer, H.G. A Hybrid Model of Tumor–Stromal Interactions in Breast Cancer. Bull Math Biol 75, 1304–1350 (2013). https://doi.org/10.1007/s11538-012-9787-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-012-9787-0