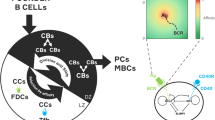
Abstract
Genetic mutations frequently observed in human follicular lymphoma (FL) B-cells result in aberrant expression of the anti-apoptotic protein bcl-2 and surface immunoglobulins (Igs) which display one or more novel variable (V) region N-glycosylation motifs. In the present study, we develop a simulation model of the germinal center (GC) to explore how these mutations might influence the emergence and clonal expansion of key mutants which provoke FL development. The simulations employ a stochastic method for calculating the cellular dynamics, which incorporates actual IgV region sequences and a simplified hypermutation scheme. We first bring our simulations into agreement with experimental data for well-characterized normal and bcl-2+ anti-hapten GC responses in mice to provide a model for understanding how bcl-2 expression leads to permissive selection and memory cell differentiation of weakly competitive B-cells. However, as bcl-2 expression in the GC alone is thought to be insufficient for FL development, we next monitor simulated IgV region mutations to determine the emergence times of key mutants displaying aberrant N-glycosylation motifs recurrently observed in human FL IgV regions. Simulations of 26 germline VH gene segments indicate that particular IgV regions have a dynamical selective advantage by virtue of the speed with which one or more of their key sites can generate N-glycosylation motifs upon hypermutation. Separate calculations attribute the high occurrence frequency of such IgV regions in FL to an ability to produce key mutants at a fast enough rate to overcome stochastic processes in the GC that hinder clonal expansion. Altogether, these simulations characterize three pathways for FL maturation through positively selected N-glycosylations, namely, via one of two key sites within germline VH region gene segments, or via a site in the third heavy chain complementarity-determining region (CDR-H3) that is generated from VDJ recombination.
Similar content being viewed by others
References
Aarts, W.M., Bende, R.J., Steenbergen, E.J., Kluin, P.M., Ooms, E.C.M., Pals, S.T., Van Noesel, C.J.M., 2000. Variable heavy chain gene analysis of follicular lymphomas: correlation between heavy chain isotype expression and somatic mutation load. Blood 95, 2922–2929.
Alabyev, B., Manser, T., 2002. Bcl-2 rescues the germinal center response but does not alter the V gene somatic hypermutation spectrum in MSH2-deficient mice. J. Immunol. 169, 3819–3824.
Allen, D., Simon, T., Sablitzky, F., Rajewsky, K., Cumano, A., 1988. Antibody engineering for the analysis of affinity maturation of an anti-hapten response. EMBO J. 7, 1995–2001.
Allen, C.D.C., Okada, T., Cyster, J.G., 2007a. Germinal-center organization and cellular dynamics. Immunity 27, 190–202. doi:10.1016/j.immuni.2007.07.009.
Allen, C.D.C., Okada, T., Tang, H.L., Cyster, J.G., 2007b. Imaging of germinal center selection events during affinity maturation. Science 315, 528–531. doi:10.1126/science.1136736.
Armitage, R.J., Sato, T.A., Macduff, B.M., Clifford, K.N., Alpert, A.R., Smith, C.A., Fanslow, W.C., 1992. Identification of a source of biologically active CD40 ligand. Eur. J. Immunol. 22, 2071–2076. doi:10.1002/eji.1830220817.
Batista, F.D., Neuberger, M.S., 1998. Affinity dependence of the B-cell response to antigen: a threshold, a ceiling, and the importance of off-rate. Immunity 8, 751–759. doi:10.1016/S1074-7613(00)80580-4.
Benson, M.J., Erickson, L.D., Gleeson, M.W., Noelle, R.J., 2007. Affinity of antigen encounter and other early B-cell signals determine B-cell fate. Curr. Opin. Immunol. 19, 275–280. doi:10.1016/j.coi.2007.04.009.
Bernasconi, N.L., Traggiai, E., Lanzavecchia, A., 2002. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 298, 2199–2202. doi:10.1126/science.1076071.
Betz, A.G., Neuberger, M.S., Milstein, C., 1993. Discriminating intrinsic and antigen-selected mutational hotspots in immunoglobulin V genes. Immunol. Today 14, 405–411. doi:10.1016/0167-5699(93)90144-A.
Bothwell, A.L.M., Paskind, M., Reth, M., Imanishi-Kari, T., Rajewsky, K., Baltimore, D., 1981. Heavy chain variable region contribution to the NPb family of antibodies—somatic mutation evident in a γ2a variable region. Cell 24, 625–637. doi:10.1016/0092-8674(81)90089-1.
Brezinschek, H.P., Brezinschek, R.I., Lipsky, P.E., 1995. Analysis of the heavy chain repertoire of human peripheral B cells using single-cell polymerase chain reaction. J. Immunol. 155, 190–202.
Brezinschek, H.P., Foster, S.J., Brezinschek, R.I., Dorner, T., Domiati-Saad, R., Lipsky, P.E., 1997. Analysis of the human VH gene repertoire. Differential effects of selection and somatic hypermutation on human peripheral CD5(+)/IgM+ and CD5(−)/IgM+ B cells. J. Clin. Invest. 99, 2488–2501. doi:10.1172/JCI119433.
Carbone, A., Gloghini, A., Gruss, H.J., Pinto, A., 1995. CD40 ligand is constitutively expressed in a subset of T-cell lymphomas and on the microenvironmental reactive T cells of follicular lymphomas and Hodgkin’s disease. Am. J. Pathol. 147, 912–922.
Cleary, M.L., Smith, S.D., Sklar, J., 1986. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell 47, 19–28. doi:10.1016/0092-8674(86)90362-4.
Cong, P., Raffeld, M., Teruya-Feldstein, J., Sorbara, L., Pittaluga, S., Jaffe, E.S., 2002. In situ localization of follicular lymphoma: description and analysis by laser capture microdissection. Blood 99, 3376–3382. doi:10.1182/blood.V99.9.3376.
Cumano, A., Rajewsky, K., 1985. Structure of primary anti-(4-hydroxy-3-nitro-phenyl)acetyl (NP) antibodies in normal and idiotypically suppressed C57BL/6 mice. Eur. J. Immunol. 15, 512–520. doi:10.1002/eji.1830150517.
Cyster, J.G., Hartley, S.B., Goodnow, C.C., 1994. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature 371, 389–395. doi:10.1038/371389a0.
Dal Porto, J.M., Haberman, A.M., Kelsoe, G., Shlomchik, M.J., 2002. Very low affinity B cells form germinal centers, become memory B cells, and participate in secondary immune responses when higher affinity competition is reduced. J. Exp. Med. 195, 1215–1221. doi:10.1084/jem.20011550.
de Wildt, R.M.T., Hoet, R.M.A., Van Venrooij, W.J., Tomlinson, I.M., Winter, G., 1999. Analysis of heavy and light chain pairings indicates that receptor editing shapes the human antibody repertoire. J. Mol. Biol. 285, 895–901. doi:10.1006/jmbi.1998.2396.
Dighiero, G., Hart, S., Lim, A., Borche, L., Levy, R., Miller, R.A., 1991. Autoantibody activity of immunoglobulins isolated from B-cell follicular lymphomas. Blood 78, 581–585.
Dogan, A., Du, M.Q., Aiello, A., Diss, T.C., Ye, H.T., Pan, L.X., Isaacson, P.G., 1998. Follicular lymphomas contain a clonally linked but phenotypically distinct neoplastic B-cell population in the interfollicular zone. Blood 91, 4708–4714.
Downing, I., Koch, C., Kilpatrick, D.C., 2003. Immature dendritic cells possess a sugar-sensitive receptor for human mannan-binding lectin. Immunology 109, 360–364. doi:10.1046/j.1365-2567.2003.01675.x.
Egle, A., Harris, A.W., Bath, M.L., O’Reilly, L., Cory, S., 2004. VavP-Bcl2 transgenic mice develop follicular lymphoma preceded by germinal center hyperplasia. Blood 103, 2276–2283. doi:10.1182/blood-2003-07-2469.
Fenwick, M.K., 2005. PhD Thesis: Modeling and Simulation of Antibody Structure and the Role Antibodies Play in the Onset of Follicular Lymphoma. Cornell University, Ithaca.
Freedman, A.S., Munro, J.M., Rice, G.E., Bevilacqua, M.P., Morimoto, C., McIntyre, B.W., Rhynhart, K., Pober, J.S., Nadler, L.M., 1990. Adhesion of human B-cells to germinal centers in vitro involves VLA-4 and INCAM-110. Science 249, 1030–1033. doi:10.1126/science.1697696.
Freedman, A.S., Munro, J.M., Morimoto, C., McIntyre, B.W., Rhynhart, K., Lee, N., Nadler, L.M., 1992. Follicular non-Hodgkin’s lymphoma cell adhesion to normal germinal centers and neoplastic follicles involves very late antigen-4 and vascular cell adhesion molecule-1. Blood 79, 206–212.
Frizzera, G., Anaya, J.S., Banks, P.M., 1986. Neoplastic plasma cells in follicular lymphomas—clinical and pathologic findings in six cases. Virchows Arch. [Pathol. Anat.] 409, 149–162.
Fukuhara, S., Rowley, J.D., Variakojis, D., Golomb, H.M., 1979. Chromosome abnormalities in poorly differentiated lymphocytic lymphoma. Cancer Res. 39, 3119–3128.
Furukawa, K., Akasako-Furukawa, A., Shirai, H., Nakamura, H., Azuma, T., 1999. Junctional amino acids determine the maturation pathway of an antibody. Immunity 11, 329–338. doi:10.1016/S1074-7613(00)80108-9.
Ghia, P., Caligaris-Cappio, F., 2000. The indispensable role of microenvironment in the natural history of low-grade B-cell neoplasms. Adv. Cancer Res. 79, 157–173. doi:10.1016/S0065-230X(00)79005-1.
Ghia, P., Boussiotis, V.A., Schultze, J.L., Cardoso, A.A., Dorfman, D.M., Gribben, J.G., Freedman, A.S., Nadler, L.M., 1998. Unbalanced expression of bcl-2 family proteins in follicular lymphoma: Contribution of CD40 signaling in promoting survival. Blood 91, 244–251.
Gillespie, D.T., 1976. A general method for numerically simulating the stochastic time evolution of coupled chemical reactions. J. Comput. Phys. 22, 403–434. doi:10.1016/0021-9991(76)90041-3.
Gray, D., Skarvall, H., 1988. B-cell memory is short-lived in the absence of antigen. Nature 336, 70–73. doi:10.1038/336070a0.
Haberman, A.M., Shlomchik, M.J., 2003. Reassessing the function of immune-complex retention by follicular dendritic cells. Nat. Rev. Immunol. 3, 757–764.
Hande, S., Notidis, E., Manser, T., 1998. Bcl-2 obstructs negative selection of autoreactive, hypermutated antibody V regions during memory B cell development. Immunity 8, 189–198. doi:10.1016/S1074-7613(00)80471-9.
Harris, N.L., 1997. Principles of the revised European-American lymphoma classification (from the international lymphoma study group). Ann. Onc. 8, 11–16. doi:10.1023/A:1008297208776.
Harris, N.L., Ferry, J.A., 1992. Follicular lymphoma and related disorders (germinal center lymphomas). In: Knowles, D.M. (Ed.), Neoplastic Hematopathology, pp. 645–674. Williams and Wilkins, Baltimore
Harris, N.L., Jaffe, E.S., Stein, H., Banks, P.M., Chan, J.K.C., Cleary, M.L., Delsol, G., De Wolf-Peeters, C., Falini, B., Gatter, K.C., Grogan, T.M., Isaacson, P.G., Knowles, D.M., Mason, D.Y., Muller-Hermelink, H.K., Pileri, S.A., Piris, M.A., Ralfkiaer, E., Warnke, R.A., 1994. A revised European-American classification of lymphoid neoplasms—a proposal from the international lymphoma study group. Blood 84, 1361–1392.
Hauser, A.E., Junt, T., Mempel, T.R., Sneddon, M.W., Kleinstein, S.H., Henrickson, S.E., Von Andrian, U.H., Shlomchik, M.J., Haberman, A.M., 2007. Definition of germinal-center B-cell migration in vivo reveals predominant intrazonal circulation patterns. Immunity 26, 655–667. doi:10.1016/j.immuni.2007.04.008.
Hockenbery, D., Nunez, G., Milliman, C., Schreiber, R.D., Korsmeyer, S.J., 1990. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 348, 334–336. doi:10.1038/348334a0.
Huang, S.C., Jiang, R., Glas, A.M., Milner, E.C.B., 1996. Non-stochastic utilization of Ig V region genes in unselected human peripheral B cells. Mol. Immunol. 33, 553–560. doi:10.1016/0161-5890(95)00162-X.
Iber, D., Maini, P.K., 2002. A mathematical model for germinal centre kinetics and affinity maturation. J. Theor. Biol. 219, 153–175. doi:10.1006/jtbi.2002.3079.
Jaffe, E.S., Shevach, E.M., Frank, M.M., Berard, C.W., Green, I., 1974. Nodular lymphoma—evidence for origin from follicular B lymphocytes. New Engl. J. Med. 290, 813–819.
Johnson, P.W., Watt, S.M., Betts, D.R., Davies, D., Jordan, S., Norton, A.J., Lister, T.A., 1993. Isolated follicular lymphoma cells are resistant to apoptosis and can be grown in vitro in the CD40/stromal cell system. Blood 82, 1848–1857.
Kepler, T.B., 1997. Codon bias and plasticity in immunoglobulins. Mol. Biol. Evol. 14, 637–643.
Kepler, T.B., Bartl, S., 1998. Plasticity under somatic mutation in antigen receptors. Curr. Top. Microbiol. Immunol. 229, 149–162.
Kepler, T.B., Perelson, A.S., 1993. Cyclic re-entry of germinal center B cells and the efficiency of affinity maturation. Immunol. Today 14, 412–415. doi:10.1016/0167-5699(93)90145-B.
Kesmir, C., De Boer, R.J., 2003. A spatial model of germinal center reactions: cellular adhesion based sorting of B cells results in efficient affinity maturation. J. Theor. Biol. 222, 9–22. doi:10.1016/S0022-5193(03)00010-9.
Kleinstein, S.H., Singh, J.P., 2003. Why are there so few key mutant clones? The influence of stochastic selection and blocking on affinity maturation in the germinal center. Int. Immunol. 15, 871–884. doi:10.1093/intimm/dxg085.sgm.
Kleinstein, S.H., Louzoun, Y., Shlomchik, M.J., 2003. Estimating hypermutation rates from clonal tree data. J. Immunol. 171, 4639–4649.
Koopman, G., Keehnen, R.M., Lindhout, E., Newman, W., Shimizu, Y., Van Seventer, G.A., de Groot, C., Pals, S.T., 1994. Adhesion through the LFA-1 (CD11a/CD18)-ICAM-1 (CD54) and the VLA-4 (CD49d)-VCAM-1 (CD106) pathways prevents apoptosis of germinal center B cells. J. Immunol. 152, 3760–3767.
Koopman, G., Keehnen, R.M.J., Lindhout, E., Zhou, D.F.H., de Groot, C., Pals, S.T., 1997. Germinal center B cells rescued from apoptosis by CD40 ligation or attachment to follicular dendritic cells, but not by engagement of surface immunoglobulin or adhesion receptors, become resistant to CD95-induced apoptosis. Eur. J. Immunol. 27, 1–7. doi:10.1002/eji.1830270102.
Kraj, P., Rao, S.P., Glas, A.M., Hardy, R.R., Milner, E.C., Silberstein, L.E., 1997. The human heavy chain Ig V region gene repertoire is biased at all stages of B-cell ontogeny, including early pre-B cells. J. Immunol. 158, 5824–5832.
Kuo, P., Bynoe, M., Diamond, B., 1999. Crossreactive B cells are present during a primary but not secondary response in BALB/c mice expressing a bcl-2 transgene. Mol. Immunol. 36, 471–479. doi:10.1016/S0161-5890(99)00052-8.
Kuppers, R., Zhao, M., Hansmann, M.L., Rajewsky, K., 1993. Tracing B-cell development in human germinal centers by molecular analysis of single cells picked from histological sections. EMBO J. 12, 4955–4967.
Kuppers, R., Klein, U., Hansmann, M.L., Rajewsky, K., 1999. Cellular origin of human B-cell lymphomas. New Engl. J. Med. 341, 1520–1529. doi:10.1056/NEJM199911113412007.
Lewis, S.M., 1994. The mechanism of V(D)J joining: lessons from molecular, immunological, and comparative analyses. Adv. Immunol. 56, 27–150. doi:10.1016/S0065-2776(08)60450-2.
Limpens, J., Stad, R., Vos, C., de Vlaam, C., de Jong, D., van Ommen, G.J., Schuuring, E., Kluin, P.M., 1995. Lymphoma-associated translocation t(14;18) in blood B cells of normal individuals. Blood 85, 2528–2536.
Lindhout, E., Mevissen, M.L.C.M., Kwekkeboom, J., Tager, J.M., de Groot, C., 1993. Direct evidence that human follicular dendritic cells (FDC) rescue germinal center B-cells from death by apoptosis. Clin. Exp. Immunol. 91, 330–336.
Liu, Y.J., Joshua, D.E., Williams, G.T., Smith, C.A., Gordon, J., Maclennan, I.C.M., 1989. Mechanism of antigen-driven selection in germinal centres. Nature 342, 929–931. doi:10.1038/342929a0.
Liu, Y.J., Cairns, J.A., Holder, M.J., Abbot, S.D., Jansen, K.U., Bonnefoy, J.Y., Gordon, J., Maclennan, I.C.M., 1991a. Recombinant 25-kDa CD23 and interleukin 1 alpha promote the survival of germinal center B cells: evidence for bifurcation in the development of centrocytes rescued from apoptosis. Eur. J. Immunol. 21, 1107–1114. doi:10.1002/eji.1830210504.
Liu, Y.J., Zhang, J., Lane, P.J.L., Chan, E.Y.T., Maclennan, I.C.M., 1991b. Sites of specific B-cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur. J. Immunol. 21, 2951–2962. doi:10.1002/eji.1830211209.
Macallan, D.C., Wallace, D.L., Zhang, Y., Ghattas, H., Asquith, B., de Lara, C., Worth, A., Panayiotakopoulos, G., Griffin, G.E., Tough, D.F., Beverley, P.C.L., 2005. B-cell kinetics in humans: Rapid turnover of peripheral blood memory cells. Blood 105, 3633–3640. doi:10.1182/blood-2004-09-3740.
Manser, T., 2004. Textbook germinal centers? J. Immunol. 172, 3369–3375.
Matsuda, F., Shin, E.K., Nagaoka, H., Matsumura, R., Haino, M., Fukita, Y., Taka-ishi, S., Imai, T., Riley, J.H., Anand, R., Soeda, E., Honjo, T., 1993. Structure and physical map of 64 variable segments in the 3’ 0.8-megabase region of the human immunoglobulin heavy chain locus. Nature Genet. 3, 88–94. doi:10.1038/ng0193-88.
McCann, K.J., Johnson, P.W.M., Stevenson, F.K., Ottensmeier, C.H., 2006. Universal N-glycosylation sites introduced into the B-cell receptor of follicular lymphoma by somatic mutation: a second tumorigenic event? Leukemia 20, 530–534. doi:10.1038/sj.leu.2404095.
Mckean, D., Huppi, K., Bell, M., Staudt, L., Gerhard, W., Weigert, M., 1984. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. Proc. Natl. Acad. Sci. U.S.A. 81, 3180–3184. doi:10.1073/pnas.81.10.3180.
Meyer-Hermann, M., 2007a. A concerted action of B-cell selection mechanisms. Adv. Complex Syst. 10, 557–580. doi:10.1142/S0219525907001276.
Meyer-Hermann, M.E., 2007b. Are T cells at the origin of B-cell lymphomas? J. Theor. Biol. 244, 656–669. doi:10.1016/j.jtbi.2006.09.006.
Meyer-Hermann, M., Deutsch, A., Or-Guil, M., 2001. Recycling probability and dynamical properties of germinal center reactions. J. Theor. Biol. 210, 265–285. doi:10.1006/jtbi.2001.2297.
Meyer-Hermann, M.E., Maini, P.K., Iber, D., 2006. An analysis of B-cell selection mechanisms in germinal centres. Math. Med. Biol. 23, 255–277. doi:10.1093/imammb/dql012.
Notidis, E., Hande, S., Manser, T., 2001. Enforced expression of bcl-2 selectively perturbs negative selection of dual reactive antibodies. Dev. Immunol. 8, 223–234. doi:10.1155/2001/83595.
Notidis, E., Heltemes, L., Manser, T., 2002. Dominant, hierarchical induction of peripheral tolerance during foreign antigen-driven B-cell development. Immunity 17, 317–327. doi:10.1016/S1074-7613(02)00392-8.
O’Connor, B.P., Vogel, L.A., Zhang, W., Loo, W., Shnider, D., Lind, E.F., Ratliff, M., Noelle, R.J., Erickson, L.D., 2006. Imprinting the fate of antigen-reactive B cells through the affinity of the B-cell receptor. J. Immunol. 177, 7723–7732.
Oeschger, S., Brauninger, A., Kuppers, R., Hansmann, M.L., 2002. Tumor cell dissemination in follicular lymphoma. Blood 99, 2192–2198. doi:10.1182/blood.V99.6.2192.
Ohm-Laursen, L., Barington, T., 2007. Analysis of 6912 unselected somatic hypermutations in human VDJ rearrangements reveals lack of strand specificity and correlation between phase II substitution rates and distance to the nearest 3’ activation-induced cytidine deaminase target. J. Immunol. 178, 4322–4334.
Oprea, M., Perelson, A.S., 1997. Somatic mutation leads to efficient affinity maturation when centrocytes recycle back to centroblasts. J. Immunol. 158, 5155–5162.
Paus, D., Phan, T.G., Chan, T.D., Gardam, S., Basten, A., Brink, R., 2006. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B-cell differentiation. J. Exp. Med. 203, 1081–1091. doi:10.1084/jem.20060087.
Petrasch, S., Kosco, M., Perez-Alvarez, C., Schmitz, J., Brittinger, G., 1992a. Proliferation of non-Hodgkin-lymphoma lymphocytes in vitro is dependent upon follicular dendritic cell-interactions. Br. J. Haematol. 80, 21–26. doi:10.1111/j.1365-2141.1992.tb06395.x.
Petrasch, S., Kosco, M., Schmitz, J., Wacker, H.H., Brittinger, G., 1992b. Follicular dendritic cells in non-Hodgkin-lymphoma express adhesion molecules complementary to ligands on neoplastic B-cells. Br. J. Haematol. 82, 695–700. doi:10.1111/j.1365-2141.1992.tb06946.x.
Petrescu, A.J., Milac, A.L., Petrescu, S.M., Dwek, R.A., Wormald, M.R., 2004. Statistical analysis of the protein environment of N-glycosylation sites: implications for occupancy, structure, and folding. Glycobiology 14, 103–114. doi:10.1093/glycob/cwh008.
Phan, T.G., Paus, D., Chan, T.D., Turner, M.L., Nutt, S.L., Basten, A., Brink, R., 2006. High affinity germinal center B cells are actively selected into the plasma cell compartment. J. Exp. Med. 203, 2419–2424. doi:10.1084/jem.20061254.
Radcliffe, C.M., Arnold, J.N., Suter, D.M., Wormald, M.R., Harvey, D.J., Royle, L., Mimura, Y., Kimura, Y., Sim, R.B., Inoges, S., Rodriguez-Calvillo, M., Zabalegui, N., de Cerio, A.L.D., Potter, K.N., Mockridge, C.I., Dwek, R.A., Bendandi, M., Rudd, P.M., Stevenson, F.K., 2007. Human follicular lymphoma cells contain oligomannose glycans in the antigen-binding site of the B-cell receptor. J. Biol. Chem. 282, 7405–7415. doi:10.1074/jbc.M602690200.
Radmacher, M.D., Kelsoe, G., Kepler, T.B., 1998. Predicted and inferred waiting times for key mutations in the germinal centre reaction: Evidence for stochasticity in selection. Immunol. Cell Biol. 76, 373–381. doi:10.1046/j.1440-1711.1998.00753.x.
Rao, S.P., Riggs, J.M., Friedman, D.F., Scully, M.S., Lebien, T.W., Silberstein, L.E., 1999. Biased VH gene usage in early lineage human B cells: Evidence for preferential Ig gene rearrangement in the absence of selection. J. Immunol. 163, 2732–2740.
Retter, I., Althaus, H.H., Munch, R., Muller, W., 2005. VBASE2, an integrative V gene database. Nucl. Acids Res. 33, D671–D674. doi:10.1093/nar/gki088.
Sablitzky, F., Wildner, G., Rajewsky, K., 1985. Somatic mutation and clonal expansion of B cells in an antigen-driven immune response. EMBO J. 4, 345–350.
Schatz, D.G., Oettinger, M.A., Schlissel, M.S., 1992. V(D)J recombination: Molecular biology and regulation. Annu. Rev. Immunol. 10, 359–383.
Schwickert, T.A., Lindquist, R.L., Shakhar, G., Livshits, G., Skokos, D., Kosco-Vilbois, M.H., Dustin, M.L., Nussenzweig, M.C., 2007. In vivo imaging of germinal centres reveals a dynamic open structure. Nature 446, 83–87. doi:10.1038/nature05573.
Shlomchik, M.J., Litwin, S., Weigert, M.G., 1990. In: Progress in Immunology. Proceedings of the Seventh International Congress of Immunology, vol. 7, p. 415.
Smith, D.S., Creadon, G., Jena, P.K., Portanova, J.P., Kotzin, B.L., Wysocki, L.J., 1996. Di- and tri-nucleotide target preferences of somatic mutagenesis in normal and autoreactive B cells. J. Immunol. 156, 2642–2652.
Smith, K.G.C., Weiss, U., Rajewsky, K., Nossal, G.J.V., Tarlinton, D.M., 1994. Bcl-2 increases memory B-cell recruitment but does not perturb selection in germinal centers. Immunity 1, 803–813. doi:10.1016/S1074-7613(94)80022-7.
Smith, K.G.C., Light, A., Nossal, G.J.V., Tarlinton, D.M., 1997. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO J. 16, 2996–3006. doi:10.1093/emboj/16.11.2996.
Smith, K.G.C., Light, A., O’Reilly, L.A., Ang, S.M., Strasser, A., Tarlinton, D., 2000. Bcl-2 transgene expression inhibits apoptosis in the germinal center and reveals differences in the selection of memory B cells and bone marrow antibody-forming cells. J. Exp. Med. 191, 475–484. doi:10.1084/jem.191.3.475.
Stamatopoulos, K., Kosmas, C., Papadaki, T., Pouliou, E., Belessi, C., Afendaki, S., Anagnostou, D., Loukopoulos, D., 1997. Follicular lymphoma immunoglobulin kappa light chains are affected by the antigen selection process, but to a lesser degree than their partner heavy chains. Br. J. Haematol. 96, 132–146. doi:10.1046/j.1365-2141.1997.8492477.x.
Stein, H., Gerdes, J., Mason, D.Y., 1982. The normal and malignant germinal center. Clin. Haematol. 11, 531–559.
Su, W., Spencer, J., Wotherspoon, A.C., 2001. Relative distribution of tumour cells and reactive cells in follicular lymphoma. J. Pathol. 193, 498–504. doi:10.1002/path.820.
Tan, T., Bogarad, L.D., Deem, M.W., 2004. Modulation of base-specific mutation and recombination rates enables functional adaptation within the context of the genetic code. J. Mol. Evol. 59, 385–399. doi:10.1007/s00239-004-2633-8.
Tomlinson, I.M., Walter, G., Marks, J.D., Llewelyn, M.B., Winter, G., 1992. The repertoire of human germline VH sequences reveals about 50 groups of VH segments with different hypervariable loops. J. Mol. Biol. 227, 776–798. doi:10.1016/0022-2836(92)90223-7.
Tsujimoto, Y., Finger, L.R., Yunis, J., Nowell, P.C., Croce, C.M., 1984. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science 226, 1097–1099. doi:10.1126/science.6093263.
Tsujimoto, Y., Cossman, J., Jaffe, E., Croce, C.M., 1985a. Involvement of the bcl-2 gene in human follicular lymphoma. Science 228, 1440–1443. doi:10.1126/science.3874430.
Tsujimoto, Y., Gorham, J., Cossman, J., Jaffe, E., Croce, C.M., 1985b. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science 229, 1390–1393. doi:10.1126/science.3929382.
Umetsu, D.T., Esserman, L., Donlon, T.A., Dekruyff, R.H., Levy, R., 1990. Induction of proliferation of human follicular (B type) lymphoma cells by cognate interaction with CD4+ T cell clones. J. Immunol. 144, 2550–2557.
Weiss, U., Rajewsky, K., 1990. The repertoire of somatic antibody mutants accumulating in the memory compartment after primary immunization is restricted through affinity maturation and mirrors that expressed in the secondary response. J. Exp. Med. 172, 1681–1689. doi:10.1084/jem.172.6.1681.
Weiss, L.M., Strickler, J.G., Medeiros, L.J., Gerdes, J., Stein, H., Warnke, R.A., 1987. Proliferative rates of non-Hodgkin’s lymphomas as assessed by Ki-67 antibody. Hum. Pathol. 18, 1155–1159. doi:10.1016/S0046-8177(87)80068-0.
Yanez, R., Barrios, Y., Cabrera, R., Diaz-Espada, F., 2006. Intraclonal variability of VH genes in follicular lymphoma patients who have received anti-idiotypic immunotherapy. J. Immunother. 29, 61–66. doi:10.1097/01.cji.0000182270.66506.e1.
Yunis, J.J., Oken, M.M., Kaplan, M.E., Ensrud, K.M., Howe, R.R., Theologides, A., 1982. Distinctive chromosomal abnormalities in histologic subtypes of non-Hodgkin’s lymphoma. New Engl. J. Med. 307, 1231–1236.
Zabalegui, N., de Cerio, A.L.D., Inoges, S., Rodriguez-Calvillo, M., Perez-Calvo, J., Hernandez, M., Garcia-Foncillas, J., Martin-Algarra, S., Rocha, E., Bendandi, M., 2004. Acquired potential N-glycosylation sites within the tumor-specific immunoglobulin heavy chains of B-cell malignancies. Haematologica 89, 541–546.
Zhu, D., McCarthy, H., Ottensmeier, C.H., Johnson, P., Hamblin, T.J., Stevenson, F.K., 2002. Acquisition of potential N-glycosylation sites in the immunoglobulin variable region by somatic mutation is a distinctive feature of follicular lymphoma. Blood 99, 2562–2568. doi:10.1182/blood.V99.7.2562.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fenwick, M.K., Escobedo, F.A. Exploration of Factors Affecting the Onset and Maturation Course of Follicular Lymphoma through Simulations of the Germinal Center. Bull. Math. Biol. 71, 1432–1462 (2009). https://doi.org/10.1007/s11538-009-9408-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-009-9408-8