Abstract
Background
Human epidermal growth factor-2 (HER2) overexpression is an oncogenic driver in many solid tumors, including urothelial bladder cancer (UBC). In addition, activating mutations in the ERBB2 gene have been shown to play an oncogenic role similar to ERBB2 amplification.
Objective
To describe and compare the frequency and nature of genomic alterations (GA) of ERBB2-altered (mutations, amplification) and ERBB2 wild-type UBC.
Patients and Methods
Using a hybrid capture-based comprehensive profiling assay, 9518 UBC cases were grouped by ERBB2 alteration and evaluated for all classes of genomic alterations (GA), tumor mutational burden (TMB), microsatellite instability (MSI), genome-wide loss of heterozygosity (gLOH), and genomic mutational signature. PD-L1 expression was measured by immunohistochemistry (Dako 22C3). Categorical statistical comparisons were performed using Fisher’s exact tests.
Results
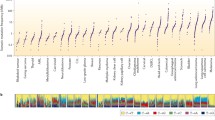
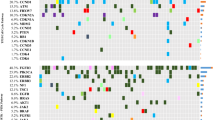
A total of 602 (6.3%) UBC cases featured ERBB2 extracellular domain short variant (SV) GA (ECDmut+), 253 (2.7%) cases featured ERBB2 kinase domain SV GA (KDmut+), 866 (9.1%) cases had ERBB2 amplification (amp+), and 7797 (81.9%) cases were ERBB2 wild-type (wt). European genetic ancestry of ECDmut+ was higher than ERBB2wt. Numerous significant associations were observed when comparing GA by group. Notably among these, CDKN2A/MTAP loss were more frequent in ERBB2wt versus ECDmut+ and amp+. ERBB3 GA were more frequent in ECDmut+ and KDmut+ than ERBB2wt. TERT GA were more frequent in ECDmut+, KDmut+, and amp+ versus ERBB2wt. TOP2A amplification was significantly more common in ECDmut+ and amp+ versus ERBB2wt, and TP53 SV GA were significantly higher in ERBB2 amp+ versus ERBB2wt. Mean TMB levels were significantly higher in ECDmut+, KDmut+, and amp+ than in ERBB2wt. Apolipoprotein B mRNA-editing enzyme, catalytic polypeptides (APOBEC) signature was more frequent in ECDmut+, KDmut+, and amp+ versus ERBB2wt. No significant differences were observed in PD-L1 status between groups, while gLOH-high status was more common in amp+ versus ERBB2wt. MSI-high status was more frequent in KDmut+ versus ERBB2wt, and in ERBB2wt than in amp+.
Conclusions
We noted important differences in co-occurring GA in ERBB2-altered (ECDmut+, KDmut+, amp+) versus ERBB2wt UBC, as well as higher mean TMB and higher APOBEC mutational signature in the ERBB2-altered groups. Our results can help refine future clinical trial designs and elucidate possible response and resistance mechanisms for ERBB2-altered UBC.

Similar content being viewed by others
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. https://doi.org/10.3322/caac.21654.
Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383:1218–30. https://doi.org/10.1056/NEJMoa2002788.
von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–8.
Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18:312–22. https://doi.org/10.1016/S1470-2045(17)30065-7.
Fradet Y, Bellmunt J, Vaughn DJ, Lee JL, Fong L, Vogelzang NJ, et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol. 2019;30:970–6. https://doi.org/10.1093/annonc/mdz127.
Apolo AB, Ellerton JA, Infante JR, Agrawal M, Gordon MS, Aljumaily R, et al. Avelumab as second-line therapy for metastatic, platinum-treated urothelial carcinoma in the phase Ib JAVELIN Solid Tumor study: 2-year updated efficacy and safety analysis. J Immunother Cancer. 2020;8: e001246. https://doi.org/10.1136/jitc-2020-001246.
Tagawa ST, Balar AV, Petrylak DP, Kalebasty AR, Loriot Y, Fléchon A, et al. TROPHY-U-01: a phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol. 2021;39:2474–85. https://doi.org/10.1200/JCO.20.03489.
Powles T, Rosenberg JE, Sonpavde GP, Loriot Y, Durán I, Lee J-L, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med. 2021;384:1125–35. https://doi.org/10.1056/NEJMoa2035807.
Yu EY, Petrylak DP, O’Donnell PH, Lee J-L, van der Heijden MS, Loriot Y, et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV-201): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2021;22:872–82. https://doi.org/10.1016/S1470-2045(21)00094-2.
Loriot Y, Matsubara N, Park SH, Huddart RA, Burgess EF, Houede N, et al. Erdafitinib or chemotherapy in advanced or metastatic urothelial carcinoma. N Engl J Med. 2023;389(21):1961–71.
van der Heijden MS, Sonpavde G, Powles T, Necchi A, Burotto M, Schenker M, et al. Nivolumab plus gemcitabine–cisplatin in advanced urothelial carcinoma. N Engl J Med. 2023;389:1778–89. https://doi.org/10.1056/NEJMoa2309863.
Powles TB, Perez Valderrama B, Gupta S, Bedke J, Kikuchi E, Hoffman-Censits J, et al. LBA6 EV-302/KEYNOTE-A39: open-label, randomized phase III study of enfortumab vedotin in combination with pembrolizumab (EV+P) vs chemotherapy (Chemo) in previously untreated locally advanced metastatic urothelial carcinoma (la/mUC). Ann Oncol. 2023;34(Suppl 2):S1340. https://doi.org/10.1016/j.annonc.2023.10.106.
Gan K, Gao Y, Liu K, Xu B, Qin W. The clinical significance and prognostic value of HER2 expression in bladder cancer: a meta-analysis and a bioinformatic analysis. Front Oncol. 2021;11: 653491. https://doi.org/10.3389/fonc.2021.653491.
Grivas PD, Day M, Hussain M. Urothelial carcinomas: a focus on human epidermal receptors signaling. Am J Transl Res. 2011;3:362–73.
Koshkin VS, O’Donnell P, Yu EY, Grivas P. Systematic review: targeting HER2 in Bladder Cancer. Bladder Cancer. 2019;5:1–12. https://doi.org/10.3233/BLC-180196.
Robertson AG, Kim J, Al-Ahmadie H, Bellmunt J, Guo G, Cherniack AD, et al. Comprehensive molecular characterization of muscle invasive bladder cancer. Cell. 2017;171:540-556.e25. https://doi.org/10.1016/j.cell.2017.09.007.
Ross JS, Wang K, Khaira D, Ali SM, Fisher HAG, Mian B, et al. Comprehensive genomic profiling of 295 cases of clinically advanced urothelial carcinoma of the urinary bladder reveals a high frequency of clinically relevant genomic alterations. Cancer. 2016;122:702–11. https://doi.org/10.1002/cncr.29826.
Ross JS, Wang K, Gay LM, Al-Rohil RN, Nazeer T, Sheehan CE, et al. A high frequency of activating extracellular domain ERBB2 (HER2) mutation in micropapillary urothelial carcinoma. Clin Cancer Res. 2014;20:68–75. https://doi.org/10.1158/1078-0432.CCR-13-1992.
Galsky MD, Del Conte G, Foti S, Yu EY, Machiels J-PH, Doger B, et al. Primary analysis from DS8201-A-U105: a phase 1b, two-part, open-label study of trastuzumab deruxtecan (T-DXd) with nivolumab (nivo) in patients (pts) with HER2-expressing urothelial carcinoma (UC). J Clin Oncol. 2022;40:438–438. https://doi.org/10.1200/JCO.2022.40.6_suppl.438.
Choudhury NJ, Campanile A, Antic T, Yap KL, Fitzpatrick CA, Wade JL, et al. Afatinib activity in platinum-refractory metastatic urothelial carcinoma in patients with ERBB alterations. J Clin Oncol. 2016;34:2165–71. https://doi.org/10.1200/JCO.2015.66.3047.
Bryce AH, Kurzrock R, Meric-Bernstam F, Hurwitz H, Hainsworth JD, Spigel DR, et al. Pertuzumab plus trastuzumab for HER2-positive metastatic urothelial cancer (mUC): preliminary data from MyPathway. J Clin Oncol. 2017;35(Suppl 6):348–348. https://doi.org/10.1200/JCO.2017.35.6_suppl.348.
Connolly R, Wang V, Hyman D, Grivas P, Mitchell E, Wright J, et al. 553P activity of trastuzumab and pertuzumab (HP) in patients with non-breast/gastroesophageal HER2-amplified tumours: results of the NCI-MATCH trial (EAY131) subprotocol. Ann Oncol. 2020;31(Suppl 4):S479–80. https://doi.org/10.1016/j.annonc.2020.08.667.
Powles T, Yu EY, Iyer G, Campbell MT, Loriot Y, De Santis M, et al. Phase 2 clinical study evaluating the efficacy and safety of disitamab vedotin with or without pembrolizumab in patients with HER2-expressing urothelial carcinoma (RC48G001). J Clin Oncol. 2023;41(Suppl 6):TPS594. https://doi.org/10.1200/JCO.2023.41.6_suppl.TPS594
RemeGen Co., Ltd. A study of RC48-ADC combined with toripalimab for first-line treatment of urothelial carcinoma. ClinicalTrials.gov identifier: NCT05302284. Updated December 18, 2023. Accessed 20 Dece 2023. https://clinicaltrials.gov/study/NCT05302284
Laé M, Couturier J, Oudard S, Radvanyi F, Beuzeboc P, Vieillefond A. Assessing HER2 gene amplification as a potential target for therapy in invasive urothelial bladder cancer with a standardized methodology: results in 1005 patients. Ann Oncol. 2010;21:815–9. https://doi.org/10.1093/annonc/mdp488.
Fleischmann A, Rotzer D, Seiler R, Studer UE, Thalmann GN. Her2 amplification is significantly more frequent in lymph node metastases from urothelial bladder cancer than in the primary tumours. Eur Urol. 2011;60:350–7. https://doi.org/10.1016/j.eururo.2011.05.035.
Bose R, Kavuri SM, Searleman AC, Shen W, Shen D, Koboldt DC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;3:224–37. https://doi.org/10.1158/2159-8290.CD-12-0349.
Mishra R, Hanker AB, Garrett JT. Genomic alterations of ERBB receptors in cancer: clinical implications. Oncotarget. 2017;8:114371–92. https://doi.org/10.18632/oncotarget.22825.
Bellmunt J, Werner L, Bamias A, Fay AP, Park RS, Riester M, et al. HER2 as a target in invasive urothelial carcinoma. Cancer Med. 2015;4:844–52. https://doi.org/10.1002/cam4.432.
Tschui J, Vassella E, Bandi N, Baumgartner U, Genitsch V, Rotzer D, et al. Morphological and molecular characteristics of HER2 amplified urothelial bladder cancer. Virchows Arch Int J Pathol. 2015;466:703–10. https://doi.org/10.1007/s00428-015-1729-4.
Schneider SA, Sukov WR, Frank I, Boorjian SA, Costello BA, Tarrell RF, et al. Outcome of patients with micropapillary urothelial carcinoma following radical cystectomy: ERBB2 (HER2) amplification identifies patients with poor outcome. Mod Pathol. 2014;27:758–64. https://doi.org/10.1038/modpathol.2013.201.
Li BT, Shen R, Buonocore D, Olah ZT, Ni A, Ginsberg MS, et al. Ado-Trastuzumab emtansine for patients with HER2-mutant lung cancers: results from a phase II basket trial. J Clin Oncol. 2018;36:2532–7. https://doi.org/10.1200/JCO.2018.77.9777.
de Martino M, Zhuang D, Klatte T, Rieken M, Rouprêt M, Xylinas E, et al. Impact of ERBB2 mutations on in vitro sensitivity of bladder cancer to lapatinib. Cancer Biol Ther. 2014;15:1239–47. https://doi.org/10.4161/cbt.29687.
Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31:1023–31. https://doi.org/10.1038/nbt.2696.
Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34. https://doi.org/10.1186/s13073-017-0424-2.
Trabucco SE, Gowen K, Maund SL, Sanford E, Fabrizio DA, Hall MJ, et al. A novel next-generation sequencing approach to detecting microsatellite instability and pan-tumor characterization of 1000 microsatellite instability-high cases in 67,000 patient samples. J Mol Diagn. 2019;21:1053–66. https://doi.org/10.1016/j.jmoldx.2019.06.011.
Sun JX, He Y, Sanford E, Montesion M, Frampton GM, Vignot S, et al. A computational approach to distinguish somatic vs. germline origin of genomic alterations from deep sequencing of cancer specimens without a matched normal. PLoS Comput Biol. 2018;14:e1005965. https://doi.org/10.1371/journal.pcbi.1005965.
Alexandrov LB, Kim J, Haradhvala NJ, Huang MN, Tian Ng AW, Wu Y, et al. The repertoire of mutational signatures in human cancer. Nature. 2020;578:94–101. https://doi.org/10.1038/s41586-020-1943-3.
Newberg J, Connelly C, Frampton G. Abstract 1599: determining patient ancestry based on targeted tumor comprehensive genomic profiling. Cancer Res. 2019;79:1599. https://doi.org/10.1158/1538-7445.AM2019-1599.
Groenendijk FH, de Jong J, Fransen van de Putte EE, Michaut M, Schlicker A, Peters D, et al. ERBB2 mutations characterize a subgroup of muscle-invasive bladder cancers with excellent response to neoadjuvant chemotherapy. Eur Urol. 2016;69:384–8. https://doi.org/10.1016/j.eururo.2015.01.014.
Wang H, Jiang Y, Jin H, Wang C. ERBB2 promoter demethylation and immune cell infiltration promote a poor prognosis for cancer patients. Front Oncol. 2022;12:1012138. https://doi.org/10.3389/fonc.2022.1012138.
Triulzi T, Forte L, Regondi V, Di Modica M, Ghirelli C, Carcangiu ML, et al. HER2 signaling regulates the tumor immune microenvironment and trastuzumab efficacy. Oncoimmunology. 2018;8: e1512942. https://doi.org/10.1080/2162402X.2018.1512942.
Kwon HJ, Park Y, Nam SK, Kang E, Kim K-K, Jeong I, et al. Genetic and immune microenvironment characterization of HER2-positive gastric cancer: their association with response to trastuzumab-based treatment. Cancer Med. 2023;12:10371–84. https://doi.org/10.1002/cam4.5769.
Griguolo G, Serna G, Pascual T, Fasani R, Guardia X, Chic N, et al. Immune microenvironment characterisation and dynamics during anti-HER2-based neoadjuvant treatment in HER2-positive breast cancer. NPJ Precis Oncol. 2021;5:23. https://doi.org/10.1038/s41698-021-00163-6.
Pal SK, Agarwal N, Choueiri TK, Stephens PJ, Ross JS, Miller VA, et al. Comparison of tumor mutational burden (TMB) in relevant molecular subsets of metastatic urothelial cancer (MUC). Ann Oncol. 2017;28(Suppl 5):V297. https://doi.org/10.1093/annonc/mdx371.004.
Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single arm, phase 2 trial. Lancet. 2016;387:1909–20. https://doi.org/10.1016/S0140-6736(16)00561-4.
Galsky MD, Saci A, Szabo PM, Azrilevich A, Horak C, Lambert A, et al. Impact of tumor mutation burden on nivolumab efficacy in second line urothelial carcinoma patients: exploratory analysis of the phase ii checkmate 275 study. Ann Oncol. 2017;28(Suppl 5):V296–7. https://doi.org/10.1093/annonc/mdx371.003.
Ross JS, Wang K, Al-Rohil RN, Nazeer T, Sheehan CE, Otto GA, et al. Advanced urothelial carcinoma: next-generation sequencing reveals diverse genomic alterations and targets of therapy. Mod Pathol. 2014;27:271–80. https://doi.org/10.1038/modpathol.2013.135.
Simon R, Atefy R, Wagner U, Forster T, Fijan A, Bruderer J, et al. HER-2 and TOP2A coamplification in urinary bladder cancer. Int J Cancer. 2003;107:764–72. https://doi.org/10.1002/ijc.11477.
Weinhold N, Jacobsen A, Schultz N, Sander C, Lee W. Genome-wide analysis of non-coding regulatory mutations in cancer. Nat Genet. 2014;46:1160–5. https://doi.org/10.1038/ng.3101.
Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA. 2013;110:6021–6. https://doi.org/10.1073/pnas.1303607110.
Borah S, Xi L, Zaug AJ, Powell NM, Dancik GM, Cohen S, et al. TERT promoter mutations and telomerase reactivation in urothelial cancer. Science. 2015;347:1006–10. https://doi.org/10.1126/science.1260200.
Arnoff TE, El-Deiry WS. CDKN1A/p21WAF1, RB1, ARID1A, FLG, and HRNR mutation patterns provide insights into urinary tract environmental exposure carcinogenesis and potential treatment strategies. Am J Cancer Res. 2021;11:5452–71.
Pal SK, Frankel PH, Mortazavi A, Milowsky M, Vaishampayan U, Parikh M, et al. Effect of cisplatin and gemcitabine with or without berzosertib in patients with advanced urothelial carcinoma. JAMA Oncol. 2021;7:1–8. https://doi.org/10.1001/jamaoncol.2021.3441.
Ascione CM, Napolitano F, Esposito D, Servetto A, Belli S, Santaniello A, et al. Role of FGFR3 in bladder cancer: treatment landscape and future challenges. Cancer Treat Rev. 2023;115: 102530. https://doi.org/10.1016/j.ctrv.2023.102530.
Bou Zerdan M, Bratslavsky G, Jacob J, Ross J, Huang R, Basnet A. Urothelial bladder cancer: genomic alterations in fibroblast growth factor receptor. Mol Diagn Ther. 2023;27:475–85. https://doi.org/10.1007/s40291-023-00647-0.
Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–22. https://doi.org/10.1038/nature12965.
Nagl NG, Patsialou A, Haines DS, Dallas PB, Beck GR, Moran E. The p270 (ARID1A/SMARCF1) subunit of mammalian SWI/SNF-related complexes is essential for normal cell cycle arrest. Cancer Res. 2005;65:9236–44. https://doi.org/10.1158/0008-5472.CAN-05-1225.
Okamura R, Kato S, Lee S, Jimenez RE, Sicklick JK, Kurzrock R. ARID1A alterations function as a biomarker for longer progression-free survival after anti-PD-1/PD-L1 immunotherapy. J Immunother Cancer. 2020;8: e000438. https://doi.org/10.1136/jitc-2019-000438.
Jana S, Brahma S, Arora S, Wladyka CL, Hoang P, Blinka S, et al. Transcriptional-translational conflict is a barrier to cellular transformation and cancer progression. Cancer Cell. 2023;41:853-870.e13. https://doi.org/10.1016/j.ccell.2023.03.021.
Denslow SA, Wade PA. The human Mi-2/NuRD complex and gene regulation. Oncogene. 2007;26:5433–8. https://doi.org/10.1038/sj.onc.1210611.
Pegoraro G, Kubben N, Wickert U, Göhler H, Hoffmann K, Misteli T. Ageing-related chromatin defects through loss of the NURD complex. Nat Cell Biol. 2009;11:1261–7. https://doi.org/10.1038/ncb1971.
Polo SE, Kaidi A, Baskcomb L, Galanty Y, Jackson SP. Regulation of DNA-damage responses and cell-cycle progression by the chromatin remodelling factor CHD4. EMBO J. 2010;29:3130–9. https://doi.org/10.1038/emboj.2010.188.
Chakrabarty A, Sanchez V, Kuba M, Rinehart C, Arteaga C. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc Natl Acad Sci USA. 2012;109:2718–23.
Serra V, Scaltriti M, Prudkin L, Eichhorn P, Ibrahim Y, Chandarlapaty S, et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene. 2011;30:2547–57.
Grivas P, Day K, Karatsinides A, Paul A, Shakir N, Owainati I, et al. Evaluation of the antitumor activity of dacomitinib in models of human bladder cancer. Mol Med. 2013;19:367–76. https://doi.org/10.2119/molmed.2013.00108.
Tamura S, Wang Y, Veeneman B, Hovelson D, Bankhead A 3rd, Broses L, et al. Molecular correlates of in vitro responses to dacomitinib and afatinib in bladder cancer. Bladder Cancer. 2018;4:77–90. https://doi.org/10.3233/BLC-170144.
Junttila T, Akita R, Parsons K, Fields C, Lewis Phillips G, Friedman L, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC0941. Cancer Cell. 2009;15:429–40.
Agus D, Akita R, Fox W, Lewis G, Higgins B, Pisacane P, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–37.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflicts of Interest (In Prior 3 Years)
J.B.L., T.E., D.R.B., A.B., and R.T. declare that they have no conflicts of interest that might be relevant to the contents of this manuscript. G.B.: no financial interests; member of NCI GU Steering Committee, Renal Task Force. J.J.: has served in a consulting/advisory role for Janssen and Pfizer. P.E.S.: no financial interests; Vice Chair of NCCN Bladder and Penile Cancer Panel. R.L.: has received research support from Predicine, Veracyte, CG Oncology, Valar Labs, and Merck; serves on clinical trial protocol committees for CG Oncology, Merck, and Janssen and has served in a consulting/advisory role for Bristol Myers Squibb, Merck, Fergene, Arquer Diagnostics, Urogen Pharma, Lucence, CG Oncology, and Janssen. A.N.: has received honoraria from Roche, MSD, AstraZeneca, Janssen, Foundation Medicine, Bristol Myers Squibb, and Astellas Pharma; has served in a consulting or advisory role for MSD, Roche, Bayer, AstraZeneca, Clovis Oncology, Janssen, Incyte, Seattle Genetics/Astellas, Bristol Myers Squibb, Rainier Therapeutics, Bicycle Therapeutics, GlaxoSmithKline, Basilea Pharmaceutica, and Catalym; has received research funding from MSD, AstraZeneca, Ipsen, and Gilead; has received travel, accommodations, and expenses from Roche, MSD, AstraZeneca, Janssen, Rainer Therapeutics, and Pfizer; and has employment and stock ownership (spouse) in Bayer. A.M.K.: has grants or contracts from FKD Therapies (now Ferring), Patient-Centered Outcomes Research Institute (PCORI), Photocure, Seagen, EnGene, Arquer Diagnostics, and SWOG; has roles on advisory board or has received consulting fees from Astellas Pharma, Biological Dynamics, Bristol Myers Squibb, CG Oncology, Cystotech, Eisai, EnGene, Ferring, Imagin Medical, Imvax, Incyte, Janssen, Medac, Merck, Nonagen Bioscience, Pfizer, Photocure, Protara Therapeutics, Roche, Seagen, Sesen Bio (now Carisma Therapeutics), Theralase, Urogen Pharma, US Biotest, and Vivet Therapeutics; holds patent for CyPRIT (Cytokine Predictors of Response to Intravesical Therapy), a joint patent with MD Anderson Cancer Center; and has leadership or fiduciary roles at European Urology Oncology, International Bladder Cancer Group (IBCG), International Bladder Cancer Network (IBCN), Journal of Urology, and UroToday. D.C.P., N.D., R.S.P.H., D.I.L. are employees of Foundation Medicine Inc., a wholly owned subsidiary of Roche, and receive equity from Roche. J.R. is an employee of Foundation Medicine Inc., a wholly owned subsidiary of Roche and receives equity from Roche, and has served in a consulting role for Tango Therapeutics and Celsius Therapeutics. P.G.: has served in a consulting role for 4D Pharma, Aadi Bioscience, Abbvie, Asieris Pharmaceuticals, Astellas, AstraZeneca, BostonGene, Bristol Myers Squibb, CG Oncology, Dyania Health, Exelixis, Fresenius Kabi, G1 Therapeutics, Genentech, Gilead Sciences, Guardant Health, ImmunityBio, Infinity Pharmaceuticals, Janssen, Lucence, Merck KGaA, Mirati Therapeutics, MSD, Pfizer, PureTech, QED Therapeutics, Regeneron, Roche, Seattle Genetics, Silverback Therapeutics, Strata Oncology, and UroGen Pharma; has received research funding from ALX Oncology, Acrivon Therapeutics, Bavarian Nordic, Bristol Myers Squibb, Debiopharm Group, Genentech, G1 Therapeutics, Gilead Sciences, GSK, Merck KGaA, Mirati Therapeutics, MSD, Pfizer, and QED Therapeutics.
Ethics Approval
Approval for this study, including a waiver of informed consent and a HIPAA waiver of authorization, was obtained from the Western Institutional Review Board (protocol no. 20152817) given the retrospective nature of this clinical study and deidentification was ensured for each tumor sample. The study was conducted in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to Participate
A waiver of informed consent and a HIPAA waiver of authorization was obtained from the Western Institutional Review Board (protocol no. 20152817) given the retrospective nature of this clinical study.
Consent for Publication
Not applicable.
Availability of Data and Material
Data are available on request from the authors.
Code Availability
Not applicable.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by D.C.P., D.R.B., and R.T. The first draft of the manuscript was written by J.B.L. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Leary, J.B., Enright, T., Bakaloudi, D.R. et al. Frequency and Nature of Genomic Alterations in ERBB2-Altered Urothelial Bladder Cancer. Targ Oncol (2024). https://doi.org/10.1007/s11523-024-01056-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s11523-024-01056-x