Abstract
Background
Recurrent or refractory solid and central nervous system (CNS) tumours in paediatric patients have limited treatment options and carry a poor prognosis. The EnGeneIC Dream Vector (EDV) is a novel nanocell designed to deliver cytotoxic medication directly to the tumour. The epidermal growth factor receptor is expressed in several CNS and solid tumours and is the target for bispecific antibodies attached to the EDV.
Objective
To assess the safety and tolerability of EGFR-Erbitux receptor EnGeneIC Dream Vector with mitoxantrone (EEDVsMit) in children with recurrent / refractory solid or CNS tumours expressing EGFR.
Patients and methods
Patients aged 2–21 years with relapsed or refractory CNS and solid tumours, or radiologically diagnosed diffuse intrinsic pontine glioma (DIPG), were treated in this phase I open-label study of single agent EEDVsMit. Thirty-seven patients’ tumours were screened for EGFR expression. EEDVsMit was administered twice weekly in the first cycle and weekly thereafter. Standard dose escalation with a rolling 6 design was employed. Dosing commenced at 5 × 108 EEDVsMit per dose and escalated to 5 × 109 EEDVsMit per dose.
Results
EGFR expression was detected in 12 (32%) of the paediatric tumours tested. Nine patients were enrolled and treated on the trial, including three patients with diffuse midline glioma. Overall, EEDVsMit was well tolerated, with no dose-limiting toxicities observed. The most common drug-related adverse events were grade 1–2 fever, nausea and vomiting, rash, lymphopaenia, and mildly deranged liver function tests. All patients had disease progression, including one patient who achieved a mixed response as the best response.
Conclusions
EGFR-Erbitux receptor targeted EnGeneIC Dream Vector with mitoxantrone can be safely delivered in paediatric patients aged 2–21 years with solid or CNS tumours harbouring EGFR expression. The discovery of EGFR expression in a high proportion of paediatric gliomas means that EGFR may be useful as a target for other treatment strategies. Targeted therapeutic-loaded EDVs may be worth exploring further for their role in stimulating an anti-tumour immune response.
ClinicalTrials.gov Identifier
NCT02687386.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A novel therapy using a nanocell to deliver chemotherapy directly to the tumour is well tolerated in paediatric patients. |
In this study one in three patients with relapsed or refractory brain tumour or solid tumours showed expression of the epidermal growth factor receptor, which can potentially be used as a therapeutic target. |
1 Introduction
Children with relapsed and refractory solid or central nervous system (CNS) tumours have limited effective treatment options and carry a poor prognosis. Traditional chemotherapeutic agents commonly used in this setting are constrained by systemic toxicity when delivered at the doses required for therapeutic benefit [1, 2]. An alternative approach to reduce toxicity to normal cells is to encapsulate the chemotherapeutic drug in a nanovector and deliver the drug intracellularly directly to tumour cells. Such “active targeting” requires a receptor at the tumour cell surface [2].
The EnGeneIC Dream Vector (EDV) is a bacterial minicell: an anucleate, non-living 400 nm diameter nanocell produced through inactivation of the genes that control normal bacterial cell division [3]. The EDV packages a chemotherapeutic agent and carries a bispecific antibody targeting specific cancer cells [2, 4]. The EDVs carry a payload of up to 1 million drug molecules, which allows a high concentration of chemotherapy to be delivered and thereby potentially increase the potency of the antitumour effects [2]. The EDVs can be targeted to receptors on cancer cells, such as EGFR, using bispecific antibodies. Following intravenous administration, the minicells extravasate into the tumour microenvironment due to leaky vasculature associated with solid tumours. The leaky vasculature results in an enhanced permeation-retention effect, which promotes accumulation of nanoparticles in tumour tissue compared to normal tissues [2, 5].
It has been demonstrated in different tumour xenograft studies that the minicells extravasate into the tumour environment via the leaky vasculature and do not extravasate into any of the other normal tissues [5]. This is likely because the minicells are 400 nm in diameter and the largest fenestrations found associated with normal vasculature are 100 nm or less [4]. EDV studies using microRNA (miRNA) also show that miRNA is delivered to tumour tissue with anti-tumoral effect in mouse models with medullary thyroid carcinoma, mesothelioma and glioblastoma [6,7,8]. Rapid accumulation of doxorubicin-containing EDVs, EEDVsDox, in the core of brain tumours was demonstrated in preclinical studies including 17 dogs with glioblastoma, using 123-iodine labelled minicells [5]. The minicells are macropinocytosed and following EDV breakdown in lysosomes, the cytotoxic drug is released into the tumour cell [2].
The EDV selectively targets the cancer cell via the bispecific antibody; one arm has specificity to the polysaccharide of the minicell and the other to the EGFR. EGFR (also known as ErbB-1) is a transmembrane tyrosine kinase and is a product of the c-erbB1 proto-oncogene [9]. EGFR is important in cell proliferation, differentiation, invasion, survival and angiogenesis [10]. EGFR is expressed in several CNS and solid tumours in adults and is associated with a poor prognosis [1, 11, 12]. EGFR expression may therefore be over-represented in patients with recurrent/refractory solid or CNS tumours and is an attractive target. The EGFR-Erbitux receptor-targeted EDV does not inhibit EGFR function, and thus requires EGFR expression for cellular internalisation, rather than intracellular signalling.
A number of clinical trials using EDVs in small and large animals have shown safety, tolerability and clinical response. Preclinical studies of EDVs packaged with mitoxantrone in murine xenograft models with colon cancer resulted in tumour stabilisation or regression [2]. EGFR-antibody linked EDVs have been shown to enter and kill paediatric neuroblastoma cells in vitro and in vivo in murine models [13, 14]. Tumour regression was shown in two dogs with advanced stage Hodgkin’s lymphoma [2]. EGFR-targeted minicells loaded with doxorubicin were administered to 17 dogs with spontaneously occurring late-stage brain cancer and clinical activity was observed, including two dogs with complete response [5].
Clinical trials have been conducted in adult patients with solid and CNS tumours. Whittle et al. describe the use of doxorubicin-containing EDVs in adult patients with recurrent glioblastoma [15]. Overall, the treatment was well tolerated, with no dose-limiting toxicity and no withdrawals from the study due to adverse events [15]. Solomon et al. described 22 adult patients who completed at least one cycle of EGFR-targeted, paclitaxel-loaded EDVs. The treatment was well tolerated and ten patients (45%; n = 22) achieved stable disease as their best response [1].
The EDV is loaded with a cytotoxic chemotherapy agent. In preclinical studies and clinical trials in adults the EDVs were loaded with the anthracycline doxorubicin. Mitoxantrone, also an anthracycline, has an established safety profile in the paediatric population and is active across a broad range of malignancies. We conducted a high throughput screen in paediatric high-grade gliomas that identified mitoxantrone as a potent anti-tumour agent in vitro. Mitoxantrone is not commonly used as a first-line therapy for paediatric solid or CNS tumours, and therefore acquired tumour resistance arising from previous exposure was considered less likely compared with doxorubicin. Therefore, mitoxantrone was selected as the anthracycline for use in this trial.
This paper describes the results of a phase I study of the use of mitoxantrone-containing EDVs targeting EGFR in paediatric patients with EGFR expressing relapsed/refractory solid or CNS tumours. We describe the proportion of patients with EGFR expression, safety and tolerability of the treatment, and preliminarily define the anti-tumour activity of mitoxantrone-containing EDVs.
2 Methods
2.1 Study Design
This was an open-label, sequential dose exploration study of single-agent EGFR-Erbitux receptor EnGeneIC Dream Vector with mitoxantrone (EEDVsMit) administered by intravenous infusion in children with recurrent/refractory solid or CNS tumours with EGFR expression or diffuse intrinsic pontine glioma (DIPG). Patients aged 2–21 years with recurrent/refractory solid/CNS tumours were enrolled, following a minimum of first-line therapy. Evidence of expression of EGFR in tumour biopsies, as assessed by immunohistochemistry (IHC), was required except in patients with DIPG who did not have a prior biopsy. Staining was reviewed by a pathologist and tumours with more than 20% of tumour cells demonstrating membranous (partial or complete) staining were scored as positive. Patients with a radiologic diagnosis of DIPG were eligible without prior biopsy. Patients were eligible for treatment only if they had fully recovered from the acute toxic effects of all prior therapies: at least 3 weeks post myelosuppressive chemotherapy; 7 days post biologic agents; 6 weeks post immunotherapy, MIBG therapy and radiotherapy; and 12 weeks post bone marrow transplant. All patients were screened for antibodies to Salmonella typhi prior to enrolment, with a positive result leading to exclusion from the study as the minicell is derived from this bacterial cell wall. Enrolment in the study required informed patient consent and the study was approved by the Sydney Children’s Hospital Network Human Research Ethics Committee (HREC). ClinicalTrials.gov Identifier: NCT02687386
The dose-exploration study used a standard dose escalation with a rolling 6 design. Dosing was guided by prior adult recurrent glioma trials; dosing commenced at one log scale below the maximum tolerated dose (MTD) in adults [15]. Dose level 0 was administered at 5 × 108 EDV per dose, dose level 1 was administered at 2.5 × 109, and dose level 2 tested the maximum dose level of 5 × 109 EDV per dose. At each dose level, the first four doses administered to each patient were administered at 10% of the target dose, before escalation to the target dose for all subsequent doses in the absence of any dose-limiting toxicities.
2.2 Treatment
Participants received EEDVsMit by intravenous injection twice weekly as a 20-min infusion beginning at study day 1 for the first 28-day cycle, then weekly for subsequent cycles. EEDVsMit contains approximately 600 μg of mitoxantrone and 5 ± 0.5 μg of anti-human EGFR (Erbitux sequence) bispecific antibody per 1 × 109 EDVs. All patients received premedication with dexamethasone, antihistamine (promethazine or loratadine) and paracetamol 30–60 min prior to the infusion. Treatment was administered until dose-limiting toxicity (DLT), disease progression, revocation of consent or cessation of trial medication production.
2.3 Toxicity Evaluation
Adverse events were graded by severity in line with the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. The relationship of the adverse event to the treatment was determined by the Investigator. DLTs were defined as clinically significant grade 3 or 4 non-haematologic toxicity, except fatigue. Nausea and vomiting were considered a DLT only if persisting longer than 7 days despite medical management. Haematologic DLTs were defined as grade 4 neutropeania, grade 3 thrombocytopaenia with bleeding or grade 4 thrombocytopaenia.
2.4 Response Evaluation
Patients underwent magnetic resonance imaging (MRI) or computed tomography (CT) assessment of their tumours at enrolment, after the second cycle of EDV therapy, and every second cycle thereafter. The modified response evaluation criteria in solid tumours (RECIST) criteria were used to assess response to treatment [16]. Blood samples were taken pre and post each cycle and peripheral blood mononuclear cells (PBMCs) were isolated. Changes in natural killer cell subsets (NK) and CD8+T cells were examined by Beckman Gallios flow cytometer using CD56, CD56dim CD16+ and CD56dim CD16- as markers for NK cells and CD45, CD3+ and CD8+ for cytotoxic T-cells.
2.5 Immunogenicity Evaluation
Serum samples pre-dose and 3 h post-dose were analysed for levels of the inflammatory cytokines tumour necrosis factor alpha (TNFα) and interleukin 6 (IL-6) following the first, second and fifth doses in cycle 1 and after the first dose only in subsequent cycles.
2.6 Statistics
Descriptive statistics were used to describe the proportion of patients with EGFR positivity and grading of adverse effects. The effect of dose level on inflammatory cytokine levels was assessed using a random-effects Tobit regression model. Event-free survival as a function of time since enrolment was measured using the Kaplan-Meier method.
3 Results
3.1 EGFR Positivity
Patients were enrolled between August 2016 and June 2018. The EGFR status of 37 patient tumours was evaluated (Table 1). EGFR testing was positive by IHC in 12 of 37 patients (32%). Most patients with EGFR-positive tumours had either high-grade gliomas or diffuse midline gliomas (DMG). Seven out of nine patients diagnosed with either high-grade glioma or glioblastoma were positive for EGFR. All patients with DMG had biopsies except for DIPG patients who were biopsied at the clinician's discretion. Four out of ten biopsied DMG patients had positive staining but were not enrolled in the trial and did not receive treatment with EEDVsMit. Notably no medulloblastoma patients were EGFR positive. Eleven patients with an extra-cranial solid tumour were tested and only one tumour was found to have EGFR expression.
3.2 Baseline Characteristics
Nine patients were enrolled in the therapeutic study and received treatment with EEDVsMit. Baseline characteristics are outlined in Tables 2 and 3. Patients were aged 5–18 years, with a median age of 9 years. Seven female and two male patients were enrolled. Five patients had a diagnosis of high-grade glioma/glioblastoma, three patients had a radiologic diagnosis of DIPG without prior biopsy, and one patient had a non-CNS solid tumour (sclerosing epithelioid fibrosarcoma). All patients were heavily pre-treated and had disease progression after previous therapy with either surgery, radiotherapy, chemotherapy, immunotherapy or a combination of these treatment modalities. The time between surgery and EDV delivery was 14–76 weeks (median 28 weeks) and time from radiotherapy to EDV delivery was 9–41 weeks (median 17 weeks). One patient had chemotherapy more than 12 months prior to EDV therapy, followed by radiotherapy 3 months prior to enrolment, and one patient had chemotherapy at least 10 weeks prior to EDV therapy. Two patients had other therapy at least 11 weeks prior to EDV therapy, including hyperthermia, vitamin infusions and immunotherapy.
3.3 Treatment Administered
Patients received treatment at three dose levels, as summarised in Table 4. Three patients received treatment at dose level 0 (5 × 108), three at dose level 1 (2.5 × 109) and three at dose level 2 (5 × 109). The minimum number of doses administered was eight doses and the maximum was 28 doses. Three patients received more than 12 doses.
3.4 Adverse Effects
A total of 171 adverse events were reported throughout the study period, of which 72 were deemed unrelated to the study drug. No DLTs were recorded. A total of 99 adverse events were deemed at least potentially related to the study drug (Table 5). The most common included fever, nausea, vomiting and rash. Biochemical abnormalities included lymphopaenia and mildly elevated transaminases.
Most adverse events were classed as grade 1 toxicity (106/170 = 62%) and only two events reached grade 4 toxicity (both lymphopenia, which was not defined as a DLT). One patient had grade 3 nausea and vomiting, which lasted less than 7 days so was not considered a DLT. Most adverse events were self-limiting, had no long-term sequelae, and led to no change in the treatment regimen. Fevers were all classified as Grade 1, most occurred immediately post infusion of the study drug, were self-limiting and not associated with systemic toxicity. Treatment with the study drug was ceased in one patient due to vomiting and headache in the setting of hydrocephalus, and this was ultimately deemed unrelated to the study drug. One patient had a temporary interruption of treatment due to facial nerve palsy, deemed unrelated to the study drug.
3.5 Immunogenicity
Antibodies to Salmonella typhi (anti-LPS) at screening were negative in all patients screened for the study.
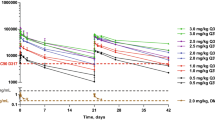
Predose TNFα was undetectable in all patients treated at all dose levels. There was an increase in TNFα at the 3 h post-dose timepoint at all dose levels (Fig. 1). The mean post dose TNFα level was 22.7 pg/mL in the patients treated at dose level 0, 71.7 pg/mL in those treated at dose level 1, and 84.5 pg/mL in those treated at dose level 2. There was a statistically significant difference between the mean TNFα at dose level 0 compared with dose level 1 (p < 0.02) and dose level 0 compared with dose level 2 (p < 0.001). The median post-treatment TNFα at dose level 1 was approximately 5.6 times higher [95% confidence interval (CI) 1.31–23.72] than that of dose level 0. The median post-treatment TNFα at dose level 2 was approximately 16.6 times higher (95% CI 3.81–72.25) than that of dose level 0. This suggests increased immunogenicity resulting from study treatment at higher dose levels.
Treatment effect on TNFα and IL-6. TNF alpha levels (A) and IL6 levels (B) were measured 3 h post administration of the study drug for patients treated at each dose level. TNFα levels significantly increased with increasing dose. The increase in IL-6 did not reach statistical significance. p values calculated by random-effects Tobit regression model
There was no detectable pre-dose IL-6 in all patients except one. There was an increase in 3-h post dose IL-6 levels at all dose levels (Fig. 1). The mean 3-h post-treatment level of IL-6 was 3479.8 at dose level 1, 6349.4 at dose level 2, and 8453.9 at dose level 3. There is no evidence for an overall difference in 3-h post-treatment IL-6 levels based on dosing level of EDV (p = 0.372). Two DMG patients had increases in naïve CD8+ T cells and cytotoxic effector T cells following dosing with EEDVsMit, as well as increases in a unique subset of NK cells, CD56dim CD16- (Fig. 2).
Naïve and effector CD8+ T cells and NK cell subtype CD56DimCD16-. The percentage of cytotoxic effector T cells in CD8+ T cells, percentage naïve T cells and CD8+ T cells and percentage CD56dim CD16- in NK cells was tested in two patients (A, B). Timepoints depicted are: 1. Screening; 2. Completion of Cycle 1; 3. Completion of Cycle 3
3.6 Outcomes
3.6.1 Tumour Response
Imaging-based tumour response data was available for five patients. All patients had progressive disease. One patient had stable disease following cycle 2 of treatment; however, they had no further imaging and had clinical disease progression. Four patients had progressive disease following cycle 2 of EDV-based therapy. One patient had progressive disease after cycle 2; however, this patient was clinically well and believed to be benefiting from treatment, so the decision was made to continue treatment. Following cycle 4, this patient had a mixed response as some lesions decreased in size and new lesions appeared. Four patients did not have repeat imaging after the baseline MRI but had clinical evidence of disease progression by the end of the first cycle.
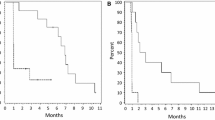
3.6.2 Mortality
All nine treated patients died within the follow-up period, as shown in Fig. 3. One patient with DIPG had a 22-month survival. She received one cycle of study drug but was removed from study due to parental concerns regarding travel constraints. The cause of death for all patients was disease progression. One patient had pneumonia recorded as a secondary cause of death. No deaths were attributed to the study medication.
4 Discussion
Treatment options for patients with relapsed/refractory solid and CNS tumours are extremely limited and these patients unfortunately have a very poor prognosis. There is a clear need to identify novel therapeutic treatment options for this high-risk patient cohort.
This phase I dose-escalation trial was the first study of bi-specific antibody-targeted cytotoxic drug-packaged minicells in a paediatric patient cohort. Our findings showed that treatment with EGFR-targeted minicells loaded with mitoxantrone is safe and well tolerated in paediatric patients. The highest planned dose level was reached, and three patients were treated at this level; however, the trial was ceased before a recommended phase 2 dose could be definitively determined due to discontinuation of the study drug. However, there were no DLTs reported, even at the higher dose levels. The most common adverse events were fever, nausea and vomiting, rash, lymphopenia, and mildly deranged liver function tests. The majority of adverse events were mild and self-limiting, and there were no treatment-related deaths.
The administration of bacterial minicells packaged with chemotherapeutic agents resulted in a mild transient rise in inflammatory cytokines. A more robust TNFα response than IL-6 response was noted. The inflammatory response may be due to the bacterial origin of the minicells [15]. Bacterial cell wall components such as lipopolysaccharides (found in the minicell outer membrane) are known to bind to receptors on the macrophage and initiate a signal transduction cascade, resulting in TNFα transcription [15]. However, TNFα production is tightly controlled to avoid pathological consequences. The anti-inflammatory cytokine IL-10 inhibits TNFα mRNA expression through activation of the STAT3 transcription factor pathway and subsequent expression of STAT3-dependent gene products [17]. In previous clinical trials, initial doses of minicells resulted in a serum spike of TNFα within physiological levels without any adverse effects, 3 h post minicell dose. At the same time points, a spike in IL-10 was observed, consistent with earlier observations that IL-10 was a moderator of TNFα. Interestingly, as subsequent minicell doses were administered, the TNFα spike gradually tapered off by doses five to ten, resulting in immune tolerance to the minicell associated LPS [18]. Immune tolerance was not observed in our study, although it is possible some patients did not receive enough treatment doses to develop immune tolerance. The induction of an immune response raises the hypothesis that EDV could be combined with immunotherapies to enhance their activity. Also, it has been shown in pre-clinical animal studies and in humans that the targeted EDV carrying a payload itself induces an innate and adaptive anti-tumour response [14]. While the cohort treated was small, immune cell analysis showed that after two cycles, two DMG patients had an increase in the CD56dim CD16- subtype of NK cells, which are a unique non-cytolytic subset that have shown correlation with better patient outcome in some tumours [19]. Naïve CD8+ T cells as well as those that had differentiated into cytotoxic effector T cells to kill cancer cells were also elevated in these patients. This response was not sustained, possibly due to an insufficient number of EDV doses administered or an insufficient dose to allow significant numbers of cytotoxic T cells to accumulate in the tumour microenvironment [14]. Future studies may benefit from using iRECIST rather than RECIST criteria to assess treatment response since targeted and drug-loaded EDVs behave as a cyto-immunotherapeutic [14].
The EGFR receptor has been identified as a viable target for targeted therapy, although its role as a target in paediatric solid and CNS tumours remains to be defined. Unlike in adult high grade glioma (HGG), EGFR is only rarely mutated or amplified in paediatric HGG; however, the proportion of CNS and solid tumours expressing EGFR as a target for conjugated antibodies has not previously been well described. In this study we observed a difference in the proportion of patients with EGFR tumours based on tumour type. EGFR positivity by IHC was observed in a high proportion of patients with a high-grade glioma or glioblastoma. The results of this study may be used in the development of further treatment options using EGFR as a target for treatment with therapies such as EDV or bispecific antibodies that rely on EGFR expression rather than mutation or amplification. Alternative EDVs are in development with mitoxantrone replaced with different payloads, which will address multi-drug resistance.
The best anti-tumour response observed in this study was a mixed response to treatment. The small numbers of patients treated at the highest dose-level may impact these findings.
This study has several limitations, particularly due to the small cohort enrolled and treated with the bacterial minicells and the early cessation of the trial. An EDV packed with a different chemotherapeutic payload may improve treatment response; however, this was not tested in this phase I trial and could be the subject of future research. Three patients with DIPG were enrolled without biopsy confirmation of EGFR positivity. It is possible this cohort did not have EGFR positivity, confounding efficacy assessment in these patients.
5 Conclusion
This study demonstrates that EEDVsMit (EGFR-Erbitux receptor targeted EnGeneIC Dream Vector with mitoxantrone) can safely be delivered in paediatric patients aged 2–21 years with relapsed or refractory solid or CNS tumours harbouring EGFR expression. The discovery of EGFR expression in a high proportion of paediatric gliomas means that EGFR may be useful as a target for EDVs carrying payloads to overcome drug resistance or for other targeted treatment strategies.
References
Solomon BJ, Desai J, Rosenthal M, McArthur GA, Pattison ST, Pattison SL, et al. A first-time-in-human phase I clinical trial of bispecific antibody-targeted, paclitaxel-packaged bacterial minicells. PLoS ONE. 2015;10: e0144559. https://doi.org/10.1371/journal.pone.0144559.
MacDiarmid JA, Madrid-Weiss J, Amaro-Mugridge NB, Phillips L, Brahmbhatt H. Bacterially-derived nanocells for tumor-targeted delivery of chemotherapeutics and cell cycle inhibitors. Cell Cycle. 2007;6:2099–105. https://doi.org/10.4161/cc.6.17.4648.
Ma L, King GF, Rothfield L. Positioning of the MinE binding site on the MinD surface suggests a plausible mechanism for activation of the Escherichia coli MinD ATPase during division site selection. Mol Microbiol. 2004;54:99–108. https://doi.org/10.1111/j.1365-2958.2004.04265.x.
MacDiarmid JA, Mugridge NB, Weiss JC, Phillips L, Burn AL, Paulin RP, et al. Bacterially derived 400 nm particles for encapsulation and cancer cell targeting of chemotherapeutics. Cancer Cell. 2007;11:431–45. https://doi.org/10.1016/j.ccr.2007.03.012.
MacDiarmid JA, Langova V, Bailey D, Pattison ST, Pattison SL, Christensen N, et al. targeted doxorubicin delivery to brain tumors via minicells: proof of principle using dogs with spontaneously occurring tumors as a model. PLoS ONE. 2016;11: e0151832. https://doi.org/10.1371/journal.pone.0151832.
Joo LJS, Weiss J, Gill AJ, Clifton-Bligh R, Brahmbhatt H, MacDiarmid JA, et al. RET kinase-regulated MicroRNA-153-3p improves therapeutic efficacy in medullary thyroid carcinoma. Thyroid. 2019;29:83–844. https://doi.org/10.1089/thy.2018.0525.
Reid G, Pel ME, Kirschner MB, Cheng YY, Mugridge N, Weiss J, et al. Restoring expression of miR-16: a novel approach to therapy for malignant pleural mesothelioma. Ann Oncol. 2013;24:3128–35. https://doi.org/10.1093/annonc/mdt412.
Khan MB, Ruggieri R, Jamil E, Tran NL, Gonzalez C, Mugridge N, et al. Nanocell-mediated delivery of miR-34a counteracts temozolomide resistance in glioblastoma. Mol Med. 2021;27:28–17. https://doi.org/10.1186/s10020-021-00293-4.
Lurje G, Lenz HJ. EGFR signaling and drug discovery. Oncology. 2009;77:400–10. https://doi.org/10.1159/000279388.
Woodburn JR. The epidermal growth factor receptor and its inhibition in cancer therapy. Pharmacol Ther. 1999;82:241–50. https://doi.org/10.1016/s0163-7258(98)00045-x.
Śledzińska P, Bebyn MG, Furtak J, Kowalewski J, Lewandowska MA. Prognostic and predictive biomarkers in gliomas. Int J Mol Sci. 2021;22(19):10373. https://doi.org/10.3390/ijms221910373.
Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. https://doi.org/10.1016/1040-8428(94)00144-i.
Sagnella SM, Trieu J, Brahmbhatt H, MacDiarmid JA, MacMillan A, Whan RM, et al. Targeted doxorubicin-loaded bacterially derived nano-cells for the treatment of neuroblastoma. Mol Cancer Ther. 2018;17:1012–23. https://doi.org/10.1158/1535-7163.Mct-17-0738.
Sagnella SM, Yang L, Stubbs GE, Boslem E, Martino-Echarri E, Smolarczyk K, et al. Cyto-immuno-therapy for cancer: a pathway elicited by tumor-targeted, cytotoxic drug-packaged bacterially derived nanocells. Cancer Cell. 2020;37:354-70.e7. https://doi.org/10.1016/j.ccell.2020.02.001.
Whittle JR, Lickliter JD, Gan HK, Scott AM, Simes J, Solomon BJ, et al. First in human nanotechnology doxorubicin delivery system to target epidermal growth factor receptors in recurrent glioblastoma. J Clin Neurosci. 2015;22:1889–94. https://doi.org/10.1016/j.jocn.2015.06.005.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Chan CS, Ming-Lum A, Golds GB, Lee SJ, Anderson RJ, Mui AL. Interleukin-10 inhibits lipopolysaccharide-induced tumor necrosis factor-α translation through a SHIP1-dependent pathway. J Biol Chem. 2012;287:38020–7. https://doi.org/10.1074/jbc.M112.348599.
van Zandwijk N, Pavlakis N, Kao SC, Linton A, Boyer MJ, Clarke S, et al. Safety and activity of microRNA-loaded minicells in patients with recurrent malignant pleural mesothelioma: a first-in-man, phase 1, open-label, dose-escalation study. Lancet Oncol. 2017;18:1386–96. https://doi.org/10.1016/s1470-2045(17)30621-6.
Vujanovic L, Chuckran C, Lin Y, Ding F, Sander CA, Santos PM, et al. CD56(dim) CD16(-) natural killer cell profiling in melanoma patients receiving a cancer vaccine and interferon-α. Front Immunol. 2019;10:14. https://doi.org/10.3389/fimmu.2019.00014.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Sydney Children’s Hospital Foundation. DSZ is supported by grants from the National Health and Medical Research Council (Synergy Grant #2019056, and Leadership Grant APP2017898) and Cancer Institute New South Wales Program Grant (TPG2037).
Conflicts of interest/competing interests
David S Ziegler reports consulting / advisory board fees from Bayer, Astra Zeneca, Accendatech, Novartis, Day One, FivePhusion, Amgen, Alexion, and Norgine and research support from Accendatech. Antoinette Anazodo reports consulting / advisory board fees from Bayer. Jennifer MacDiarmid and Himanshu Brahmbhatt are shareholders in EnGeneIC. Louise Evans, Rick Walker, Geoffrey McCowage, Andrew J Gifford, Maria Kavallaris, and Toby Trahair declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics approval
The study was approved by the Sydney Children’s Hospital Network Human Research Ethics Committee (HREC). ClinicalTrials.gov Identifier: NCT02687386.
Consent to participate
Enrolment on the study required informed written patient or parental consent
Consent for publication
Consent to enrolment on study included consent for publication.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Author contributions
David S. Ziegler, Jennifer MacDiarmid, Himanshu Brahmbhatt, Rick Walker, Antoinette Anazodo, Geoffrey McCowage, Andrew J. Gifford, Maria Kavallaris and Toby Trahair contributed to the study conception and design. Material preparation, data collection and analysis were performed by Louise Evans and David S. Ziegler. The first draft of the manuscript was written by Louise Evans and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Evans, L., Walker, R., MacDiarmid, J. et al. A Phase 1 Study of Intravenous EGFR-ErbituxEDVsMIT in Children with Solid or CNS Tumours Expressing Epidermal Growth Factor Receptor. Targ Oncol 19, 333–342 (2024). https://doi.org/10.1007/s11523-024-01051-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-024-01051-2