Abstract
Background
Non-inferiority of trifluridine/tipiracil (FTD/TPI) plus bevacizumab (BEV) to irinotecan/fluoropyrimidine plus BEV in metastatic colorectal cancer was investigated in the phase III TRUSTY study, and we conducted a phase II study of FOLFIRI (5-FU+leucovorin+irinotecan) plus zib-aflibercept (AFL) after FTD/TPI plus BEV. However, the TRUSTY study failed during the recruitment of our patients.
Objective
We present the findings of a phase II study on the efficacy of FOLFIRI plus zib-aflibercept (AFL) after FTD/TPI plus BEV, including clinical results with plasma biomarker analyses.
Methods
This was a multicenter, single-arm, phase II study in patients with metastatic colorectal cancer refractory or intolerant to oxaliplatin, fluoropyrimidine, BEV, and FTD/TPI. The primary endpoint was progression-free survival. Fifteen plasma angiogenesis-associated biomarkers were analyzed using a Luminex® multiplex assay U-kit.
Results
Between January 2020 and May 2022, 26 patients (median age, 68 years) from 15 sites were enrolled. The median progression-free survival was 4.9 months (85% confidence interval, 3.4 month–not estimated). The overall response and disease control rates were 8% and 62%, respectively. The median levels of vascular endothelial growth factor-A and placental growth factor, both targets of AFL, were below the measurable limit of 30 pg/mL and 16 pg/mL, respectively. Patients were divided into two groups at the median levels of baseline biomarkers. The progression-free survival did not differ between high and low expressers of placental growth factor (p = 0.7), while it tended to be shorter in those with high levels of osteopontin (p = 0.05), angiopoietin-2 (p = 0.07), and tissue inhibitor of matrix metalloproteinases-1 (p = 0.1).
Conclusions
This study did not meet the primary endpoint. Hence, FOLFIRI plus AFL should not be used after FTD/TPI plus BEV for metastatic colorectal cancer. Further studies are needed to determine factors not targeted by AFL that may affect the efficacy of the treatment.
Clinical Trial Registration
jRCTs041190100.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Patients who received FOLFIRI (5-FU+leucovorin+irinotecan) plus zib-aflibercept had a median progression-free survival of 4.9 months. |
FOLFIRI plus aflibercept after trifluridine/tipiracil plus bevacizumab did not improve survival in patients with metastatic colorectal cancer. |
However, some angiogenic biomarkers tended to be correlated with better efficacy of FOLFIRI plus aflibercept, and FOLFIRI plus aflibercept may be effective regardless of the levels of placental growth factor. |
1 Introduction
Ziv-aflibercept (AFL) is a potent anti-angiogenic agent that binds to circulating vascular endothelial growth factor (VEGF)-A, VEGF-B, and placental growth factor (PlGF), suppressing endothelial cell proliferation. Compared to bevacizumab (BEV), AFL binds more potently to VEGF-A. It also blocks PlGF and suppresses the growth of BEV-resistant tumors [1]. Moreover, the switching from BEV to AFL induced higher tumor suppression than continuing BEV in mouse xenograft models of colorectal cancer [2]. Clinically, the phase III VELOUR study demonstrated the survival benefit of FOLFIRI (5-FU+leucovorin+irinotecan) plus AFL compared with that of FOLFIRI in patients with metastatic colorectal cancer (mCRC) after the failure of first-line chemotherapy [3]. The post-hoc circulating biomarker analysis in the VELOUR study suggested that in patients with high baseline plasma VEGF-A and PlGF levels, the progression-free survival (PFS) and overall survival (OS) could be improved with FOLFIRI plus AFL therapy [4]. However, this has not been validated in another clinical study. Furthermore, the dynamics of angiogenesis-associated biomarkers after AFL administration and acquired resistance to AFL remain unclear.
Trifluridine/tipiracil (FTD/TPI) monotherapy is a standard third-line or later-line chemotherapy for mCRC [5]. Trifluridine/tipiracil plus BEV has shown promising efficacies in several phase II studies [6,7,8,9,10,11]. Based on these reports, a phase III study (TRUSTY) was initiated in October 2017, which investigated the non-inferiority of FTD/TPI plus BEV to fluoropyrimidine (FP) plus irinotecan (IRI) plus BEV in patients with mCRC refractory to oxaliplatin (OX)-based chemotherapy plus BEV [12]. Anticipating that FTD/TPI plus BEV would be a second-line treatment option instead of FOLFIRI plus anti-VEGF agents, we started a phase II study to evaluate the efficacy and safety of third-line FOLFIRI plus AFL therapy in patients with mCRC previously treated with OX, FP, BEV, and FTD/TPI from January 2020. However, the TRUSTY study was discontinued in October 2020 because it did not demonstrate the non-inferiority of FTD/TPI plus BEV to FP plus IRI plus BEV in OS [12]. Therefore, our phase II study had to be completed with a reduced sample size.
In this paper, we report the exploratory results of efficacy and safety for the third-line FOLFIRI plus AFL therapy, focusing on plasma angiogenesis-associated biomarkers, including VEGF-A and PlGF.
2 Patients and Methods
2.1 Study Design
This multicenter, single-arm, prospective phase II study was conducted with the support of the West Japan Oncology Group in compliance with the Declaration of Helsinki and Japanese ethical guidelines for clinical studies. Written informed consent was obtained from all patients before study enrollment. This study was approved by the certified Review Board of Aichi Cancer Center Hospital and has been registered in the Japan Registry of Clinical Trials (jRCTs041190100).
2.2 Patients
The full inclusion and exclusion criteria have been described in the study protocol (in the ESM). Eligible patients were aged ≥20 years with histologically confirmed metastatic colorectal adenocarcinoma, had a measurable lesion by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, and were refractory or intolerant to OX, FP, BEV, and FTD/TPI.
2.3 Treatment Procedures
The FOLFIRI plus AFL regimen consisted of a drip infusion of AFL (4 mg/kg), IRI (150 mg/m2), l-leucovorin (200 mg/m2), and a bolus infusion of 5-FU (400 mg/m2) followed by a 46-hour continuous infusion of 5-FU (2400 mg/m2). The treatment was repeated every 2 weeks until progressive disease (PD), unacceptable toxicity, or withdrawal of consent. The IRI dose was reduced to 120 mg/m2 when patients had the following gene polymorphisms: homozygous UGT1A1*28 or UGT1A1*6, or heterozygous UGT1A1*28 and UGT1A1*6. The dose reduction plan has been described in the study protocol (in the ESM).
2.4 Responses and Adverse Events
Tumor assessments by computed tomography or magnetic resonance imaging were repeated every 8 weeks. Tumor responses were evaluated according to RECIST version 1.1. Adverse events were evaluated every 2 weeks according to the Common Terminology Criteria for Adverse Events version 5.0.
2.5 Endpoints
The primary endpoint was PFS, defined as the time from enrollment to disease progression or death from any cause. The secondary endpoints were OS, overall response rate (ORR), disease control rate (DCR), and safety. Overall survival was defined as the time from enrollment to death from any cause. Overall response rate was defined as the proportion of patients achieving a complete or partial response, and DCR was defined as the proportion of patients achieving a complete/partial response or stable disease. All endpoints were analyzed in eligible patients who received at least one dose of FOLFIRI plus AFL.
2.6 Biomarker Analyses
Plasma samples were collected at three timepoints: (i) before treatment (baseline); (ii) 29 days after the initiation of therapy; and (iii) within 30 days after the discontinuation of therapy and before the post-trial therapy. The Luminex® technology-based multiplex assay U-kit was used to measure 17 biomarkers: hepatocyte growth factor, PlGF, VEGF-A, VEGF-D, interferon-γ, interleukin (IL)-6, IL-8, angiopoietin-2 (Ang-2), neuropilin-1, thrombospondin-2, osteopontin (OPN), soluble vascular epithelial growth factor receptor 1 (sVEGFR1), sVEGFR2, sVEGFR3, soluble intercellular adhesion molecule 1, soluble vascular cell adhesion molecule 1, and tissue inhibitor of matrix metalloproteinase-1. The results for sVEGFR1 and sVEGFR2 obtained after AFL administration were excluded from the analyses because they might have been affected by the VEGFR1 and VEGFR2 domains of AFL.
2.7 Statistical Analysis
In previous clinical studies on the third-line or later-line chemotherapy for patients with mCRC, the median PFS was 3.7 months with FTD/TPI plus BEV [6] and 4.1 months with IRI plus cetuximab [13]. In the VELOUR study, which was a second-line chemotherapy trial, the median PFS was 6.9 months with FOLFIRI plus AFL [3]. On the basis of these results, the null hypothesis for median PFS was set at 3.9 months, and the alternative hypothesis for median PFS was set at 6.2 months. The null hypothesis was tested using normal approximation [14]. Initially, the required sample size was calculated to be at least 46 patients to preserve a power of 0.80 with a one-sided significance level of 0.05. Considering possible dropouts, the target sample size was 50 patients.
When the enrollment of the TRUSTY study was terminated because of futility on 1 October, 2020, 17 patients had been enrolled, after which the patient accrual became slower than the planned schedule. At that point, the protocol was amended to decrease the number of patients to 25 to preserve a power of 0.75 with a one-sided alpha of 0.15. The same certified review board approved the amended protocol on 23 August, 2021.
All the analyses were performed using SAS (version 9.4) and JMP version 12.2 (SAS Institute, Cary, NC, USA). Both PFS and OS were estimated using the Kaplan–Meier method. Two-sided confidence intervals (CIs) for the median values were calculated using the Brookmeyer–Crowley method. In biomarker analyses, the cut-off values for each marker to divide the patients into two groups were set at the median. Changes in the plasma biomarker levels during the treatment were analyzed by one-way analysis of variance. Given the exploratory nature of this study, the significance level was set at ≤ 0.1, and the adjustment of multiple comparisons was not performed.
3 Results
3.1 Patient Characteristics
Between January 2020 and May 2021, a total of 26 eligible patients were enrolled. The baseline characteristics of these patients are summarized in Table 1. The patients’ median age was 68 (range 47–80) years. Ten patients (38%) were male and 15 patients (58%) had ECOG PS 1, eight (31%) had right-sided tumors, 18 (69%) had metastases in two or more organs, 12 (46%) had RAS mutations, and six (23%) had RAS wild-type tumors and had been previously treated with anti-EGFR inhibitors. Median time from the start of initial treatment for metastatic disease to enrollment in this study was 21 months (range 9.6–75 months).
3.2 Efficacy
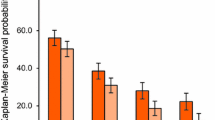
The data cut-off was on 31 May, 2022, with a median follow-up of 18 months (95% CI 15–23). The median PFS was 4.9 months (85% CI 3.4–not estimated) (Fig. 1a). The lower limit of the 85% CI for the median PFS was lower than the threshold of 3.9 months, and therefore, this study did not meet the primary endpoint. The median OS was 13 months (95% CI 8.3–17) (Fig. 1b). The ORR was 8% (95% CI 0.9–25) and the DCR was 62% (95% CI 41–80) (Table 2). Median relative dose intensity was 73% for AFL, 69% for IRI, 38% for bolus 5-FU, and 68% for continuous 5-FU. The treatment was discontinued because of disease progression in 24 patients (92%) and adverse events in two patients (cerebral infarction and respiratory failure). Subsequent treatments administered to 20 patients (77%), which included regorafenib to seven patients (27%), an anti-EGFR antibody-containing regimen to five patients (19%), and other regimens to eight patients (31%).
3.3 Safety
The adverse events are shown in Table 3. The common grade 3 or 4 adverse events were neutropenia in 11 patients (42%), reduced white blood cell count in six patients (23%), anemia and fatigue in five patients (19%) each, appetite loss and hypertension in three patients (12%) each, proteinuria and thromboembolism in one patient (4%) each. While there were no cases of febrile neutropenia, grade 5 treatment-related adverse events (respiratory failure) occurred in one patient (4%). Cycle delays were reported in 23 patients (88%) and dose modifications for AFL, IRI, and 5-FU were required in seven patients (27%), 17 patients (65%), and 19 patients (73%), respectively. Major reasons for dose modification were proteinuria (86%) for AFL and neutropenia (63%) for IRI and 5-FU.
3.4 Biomarker Analyses
Pretreatment samples were collected for biomarker analyses in 25 patients (96%). The median values of five pretreatment biomarkers (VEGF-A, VEGFR1, VEGER3, interferon-γ, and IL-6) were lower than the detectable limit. The PFS was compared between patients with baseline high and low levels of 12 biomarkers, which were Ang-2, hepatocyte growth factor, PlGF, VEGF-D, OPN, neuropilin-1, VEGFR2, IL-8, TIMP-1, thrombospondin-2, soluble intercellular adhesion molecule, and soluble vascular cell adhesion molecule 1. Patients with high and low PlGF levels (median PFS 4.0 vs 5.4 months, p = 0.7) showed no difference in PFS. However, differences were seen in the PFS of patients with high and low levels of Ang-2 (median PFS 2.9 vs 9.0 months, p = 0.07), OPN (median PFS 3.3 vs 5.5 months, p = 0.05), and TIMP-1 (median PFS 3.3 vs 6.2 months, p = 0.1) (Table 4). No biomarkers correlated with ORR and DCR (Table 1 of the Electronic Supplementary Material [ESM]).
Biomarker samples on day 29 and PD were obtained from 21 and 20 patients, respectively. Analyses of biomarkers collected at the three timepoints revealed remarkable increases (p < 0.05) in VEGF-A (p = 0.007), PlGF (p < 0.001), and soluble vascular cell adhesion molecule 1 (p = 0.007) (Fig. 2). There were no significant differences in DCR between high and low levels of day-29 VEGF-A (75% and 62%, respectively, p = 0.52) and day-29 PlGF (64% and 70%, respectively, p = 0.76). No remarkable changes were seen in the other biomarkers (Fig. 1 of the ESM).
4 Discussion
The majority of our patients (96%) were those in whom second-line FTD/TPI plus BEV therapy for mCRC had failed in the TRUSTY study, wherein the therapy did not demonstrate its non-inferiority to FOLFIRI plus BEV in terms of OS and therefore was no longer used before FOLFIRI plus BEV therapy [12]. Moreover, our study of FOLFIRI plus AFL therapy after FTD/TPI plus BEV did not meet the primary endpoint for PFS. These results support the idea that FOLFIRI plus AFL should be recommended as a second-line therapy. Considering that BEV showed an additional efficacy to FTD/TPI in the third-line setting [6, 7], it is not clear why the therapeutic effect of AFL was not as good as expected in this study. A possible reason for the relatively poor prognosis may be because of the long time from the initial treatment to enrollment with a median of 21 months, suggesting the long duration of BEV exposure. Furthermore, it is also concerned that the IRI dose of 150 mg/m2 (global standard dose, 180 mg/m2) used in this study caused the negative results. However, in our previous phase III study (WJOG4407) comparing first-line FOLFIRI (IRI 150 mg/m2)+BEV and FOLFOX+BEV, FOLFIRI+BEV showed a slightly better tendency of efficacy than FOLFOX+BV, suggesting that this difference of 30 mg/m2of IRI did not have a substantial impact on efficacy [15]. Therefore, it is unlikely that the dose of IRI at 150 mg/m2caused the negative results of this study.
In the VELOUR study, the frequency of all grade/≥ grade 3 proteinuria was 62.2%/7.8%, and all grade/≥ 3 grade hypertension was 41.4%/19.3% [3]. In comparison, in our study, all grade adverse events were almost similar, but > grade 3 adverse events were generally less frequent. Approximately 30% of patients in the VELOUR study received prior BEV, while all patients received it in our study. Possible reasons for this could be that compared to the VELOUR study, our study had a shorter PFS, and was conducted approximately 12 years later, when improved management of BEV-related proteinuria and hypertension was prevalent. However, ≥ grade 3 anorexia (12% vs 3.4%) and fatigue (19% vs 16.8%), and dose modification of IRI (37% vs 65%) and 5-FU (39% vs 73%) tended to be more common in our study than in the VELOUR study. These results might be because of the differences in patient backgrounds caused by the different treatment lines. The median age was 61 years, and 57.8% of patients had two or more metastatic organs in the VELOUR study of second-line treatment [3], while the median age was 68 years, and 69% had two or more metastatic organs in our study of third-line treatment.
For the biomarker analyses, we used the U-kit, a fluorescence-based assay that measures multiple angiogenesis-associated factors simultaneously. In vitro experiments showed that levels of VEGF and PlGF decreased in response to the addition of AFL (data not shown). Therefore, plasma samples collected during AFL therapy were assayed for unbound (free) forms of VEGF-A and PlGF. High plasma VEGF-A levels have been reported to decrease to lower than the measurable levels (<30 pg/mL) at disease progression with the first-line BEV-containing chemotherapy [16]. Similarly, the baseline levels of free VEGF-A were low in our patients, probably because of its binding to BEV used in the prior treatments. Substantial elevation in VEGF-A levels was observed in some patients on day 29 and at the time of disease progression, although the levels still appeared to be suppressed in many patients (Fig. 2). This increase in VEGF-A levels should be studied further in relation to the plasma trough concentration of AFL and its association with tumor progression. Placental growth factor levels were significantly elevated on day 29 despite FOLFIRI plus AFL therapy, indicating the inadequate suppression of circulating PlGF, which could be a cause for this study’s failure.
In contrast to our findings, the VELOUR study reported elevated plasma VEGF-A levels in patients who received prior BEV-containing chemotherapy [17]. It also showed an improvement in PFS and OS in patients with high VEGF-A levels (> 144 pg/mL), almost to the levels seen in patients with low VEGF-A levels in the FOLFIRI plus AFL arm [17]. This discrepancy could be because the VELOUR study probably measured VEGF-A levels, including the BEV-binding form, which was considered to reflect the total VEGF-A production from the tumor. Another study suggested that the BEV-VEGF-A complex accumulates in the blood during BEV-combined treatments because of a decrease in its endothelial clearance [18]. However, we could not assess VEGF levels in the same way because the U-kit measured only the free VEGF-A, and its baseline levels were very low. Hence, differences in the assays measuring free and bound forms of VEGF-A may affect the results, and this should be taken into consideration in future investigations.
We found no significant differences in the PFS and OS between patients with high and low plasma levels of PlGF, consistent with the VELOUR study. The baseline PlGF levels were relatively high in our study (median 16 pg/mL) than in the VELOUR study (median 8 pg/mL) [17]. Patients with high PlGF levels reportedly had significantly worse PFS following chemotherapy plus BEV [19]. These findings suggest that the effect of AFL is not correlated with PlGF levels.
Among other biomarkers, OPN, Ang-2, and TIMP-1 tended to correlate with PFS. These have already been reported as candidate predictive biomarkers for the efficacy of anti-angiogenesis therapy for mCRC [20,21,22,23,24,25,26,27]. Furthermore, OPN, Ang-2, and TIMP-1 have been reported to be prognostic factors for colorectal cancer [22, 26, 28]. A remarkable elevation in VCAM-1 levels has been reported for FOLFIRI plus AFL therapy [24]. These factors could be potential targets for new agents that could be added to or given after AFL therapy.
Our study has several limitations. First, the study design included a single treatment arm and had a small sample size. FOLFIRI plus AFL as a third-line treatment has little clinical significance after the TRUSTY study. Second, a U-kit was used for the biomarker analyses, which might have produced inconsistent findings. However, feasible results using this kit have been already reported in a few papers [16] [19] [29]. Third, while the median value was used as the cut-off for each angiogenesis factor, it may not be easy to establish the appropriate cut-off values in different treatments. Finally, we did not include a validation cohort, and the multiple comparisons of the biomarker analyses were not adjusted because of the exploratory nature of our study.
5 Conclusions
FOLFIRI plus AFL after FTD/TPI plus BEV did not improve survival in patients with mCRC. Further, OPN, Ang-2, and TIMP-1 levels could be predictive indicators of PFS following FOLFIRI plus AFL treatment.
References
Patel A, Sun W. Ziv-aflibercept in metastatic colorectal cancer. Biologics. 2014;8:13–25.
Chiron M, Bagley RG, Pollard J, Henry C, Mankoo LV, Vincent L, et al. Switching to aflibercept treatment resulted in greater tumor responses than continuous bevacizumab treatment in patient-derived xenograft models of colorectal cancer. In: Proceedings of the 2013 AACR-NCI-EORTC International Conference on Molecular Targets. Mol Cancer Ther. 2013;12:11:Abstract B2.
Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausova J, Macarrulla T, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499–506.
Sims TN, Gao B, Chiron M, Mancini P, Bagley R, Lowy I. Potential predictive and prognostic biomarkers identified in baseline plasma samples from the VELOUR trial. J Clin Oncol. 2015;33(3_Suppl.638).
Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunama N, et al. Randomized trial of TAS–102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909–19.
Kuboki Y, Nishina T, Shinozaki E, Yamazaki K, Shitara K, Okamoto W, et al. TAS-102 plus bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (C-TASK FORCE): an investigator-initiated, open-label, single-arm, multicentre, phase 1/2 study. Lancet Oncol. 2017;18:1172–81.
Pfeiffer P, Yilmaz M, Möller S, Zitnjak D, Krogh M, Peterson LN, et al. TAS-102 with or without bevacizumab in patients with chemorefractory metastatic colorectal cancer: an investigator-initiated, open-label, randomised, phase 2 trial. Lancet Oncol. 2020;21:412–20.
Takahashi T, Yamazaki K, Oki E, Shiozawa M, Mitsugi K, Makiyama A, et al. Phase II study of trifluridine/tipiracil plus bevacizumab by RAS mutation status in patients with metastatic colorectal cancer refractory to standard therapies: JFMC51-1702-C7. ESMO Open. 2021;6: 100093.
Ishizaki T, Mazaki J, Enomoto M, Shigoka M, Kasahara K, Matsudo T, et al. Prospective multicenter phase II study of biweekly TAS-102 and bevacizumab for metastatic colorectal cancer. Anticancer Res. 2021;41:2157–63.
Satake H, Kato T, Oba K, Kotaka M, Kagawa Y, Yasui H, et al. Phase Ib/II study of biweekly TAS-102 in combination with bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (BiTS study). Oncologist. 2020;2512:e1855–63.
Yoshida Y, Yamada T, Kamiyama H, Kosugi C, Ishibashi K, Yoshida H, et al. Combination of TAS-102 and bevacizumab as third-line treatment for metastatic colorectal cancer: TAS-CC3 study. Int J Clin Oncol. 2021;26:111–7.
Kuboki Y, Terazawa T, Masuishi T, Nakamura M, Watanabe J, Ojima H, et al. Trifluridine/tipiracil+bevacizumab (BEV) vs. fluoropyrimidine irinotecan+BEV as second-line therapy for metastatic colorectal cancer: a randomised noninferiority trial. Br J Cancer. 2023;128:1897–905.
Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45.
Lawless, Jerald F. Statistical models and methods for lifetime data. 2nd ed. Manhattan: Wiley; 2003. (equation 4.1.5).
Yamazaki K, Nagase M, Tamagawa H, Ueda S, Tamura T, Murata K, et al. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G). Ann Oncol. 2016;27:1539–46.
Yuki S, Yamazaki K, Sunakawa Y, Taniguchi H, Bando H, Shiozawa M, et al. Role of plasma angiogenesis factors in the efficacy of first-line chemotherapy combined with biologics in RAS wild-type metastatic colorectal cancer: results from the GI-SCREEN CRC-Ukit study. Cancer Med. 2023;12:18702–16.
Van Cutsem E, Paccard C, Chiron M, Tabernero J. Impact of prior bevacizumab treatment on VEGF-A and PlGF levels and outcome following second-line aflibercept treatment: biomarker post hoc analysis of the VELOUR trial. Clin Cancer Res. 2020;26:717–25.
Alidzanovic L, Starlinger P, Schauer D, Maier T, Feldoman A, Buchberger E, et al. The VEGF rise in blood of bevacizumab patients is not based on tumor escape but a host-blockade of VEGF clearance. Oncotarget. 2016;7:57197–212.
Yuki S, Taniguchi H, Masuishi T, Shiozawa M, Bando H, Yamazaki K, et al. Impact of plasma angiogenesis factors on the efficacy of 2nd line chemotherapy combined with biologics in metastatic colorectal cancer (mCRC); early efficacy results from GI SCREEN CRC Ukit study. Ann Oncol. 2021;32:S563–4.
Kariya Y, Kariya Y. Osteopontin in cancer: mechanisms and therapeutic targets. Int J Transl Med. 2022;2:419–47.
Assidi M, Gomaa Y, Jafri M, Hanbazazh M, Al-Ahwal M, Pushparaj P, et al. Prognostic value of osteopontin (SPP1) in colorectal carcinoma requires a personalized molecular approach. Tumour Biol. 2019;41:1010428319863627.
Zhao M, Liang F, Zhang B, Yan W, Zhang J. The impact of osteopontin on prognosis and clinicopathology of colorectal cancer patients: a systematic meta-analysis. Sci Rep. 2015;5:12713.
Amilca-Seba K, Sabbah M, Larsen AK, Denis JA. Osteopontin as a regulator of colorectal cancer progression and its clinical applications. Cancers (Basel). 2021;28(13):3793.
Hamaguchi T, Denda T, Kudo T, Sugimoto N, Ura T, Yamazaki K, et al. Exploration of potential prognostic biomarkers in aflibercept plus FOLFIRI in Japanese patients with metastatic colorectal cancer. Cancer Sci. 2019;110:3565–72.
Kim H, Ahn ST, Kim JC, Bae SB, Kim HJ, Lee CS, et al. Oncogenic function of angiopoietin-2 in vitro and its modulation of tumor progression in colorectal carcinoma. Oncol Lett. 2017;14:553–60.
Goede V, Coutelle O, Neuneier J, Reinacher-Schick A, Schnell R, Koslowsky TC, et al. Identification of serum angiopoietin-2 as a biomarker for clinical outcome of colorectal cancer patients treated with bevacizumab-containing therapy. Br J Cancer. 2010;103:1407–14.
Sorensen NM, Bystrom P, Christensen IJ, Berglund A, Nielsen HJ, Brunner N, et al. TIMP-1 is significantly associated with objective response and survival in metastatic colorectal cancer patients receiving combination of irinotecan, 5-fluorouracil, and folinic acid. Clin Cancer Res. 2007;13:4117–22.
Böckelman C, Beilmann-Lehtonen I, Kaprio T, Koskensalo S, Tervahartiala T, Mustonen H, et al. Serum MMP-8 and TIMP-1 predict prognosis in colorectal cancer. BMC Cancer. 2018;18:679.
Sunakawa Y, Kuboki Y, Watanabe J, Terazawa T, Kawakami H, Yokota M, et al. Exploratory biomarker analysis using plasma angiogenesis-related factors and cell-free DNA in the TRUSTY study: a randomized, phase II/III study of trifluridine/tipiracil plus bevacizumab as second-line treatment for metastatic colorectal cancer. Target Oncol. 2024;19:59–69.
Acknowledgments
We thank the participants and their families, as well as the study investigators and study teams, for their contribution. This study was supported by the West Japan Oncology Group, a non‐profit organization, and collaborated with G&G Science, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by Sanofi Co. Ltd.
Conflicts of Interest/Competing Interests
Toshihiko Matsumoto received research funding from Ono Pharmaceutical Co., Ltd and Sanofi Co., Ltd; honoraria from Bayer Co., Ltd, Bristol-Myers Squibb Co., Ltd, Chugai Pharmaceutical Co., Ltd, Daiichi Sankyo Co., Ltd, Eli Lilly Japan Co., Ltd, Merck Bio Pharma Co., Ltd, MSD Co., Ltd, Ono Pharmaceutical Co., Ltd, Sanofi Co, Ltd, Taiho Pharmaceutical Co., Ltd, Takeda Co., Ltd, Teijin Pharmaceutical Co., Ltd, and Yakult Honsha Co., Ltd. Yoshiyuki Yamamoto received honoraria for lectures from Sanofi Co. Ltd, Ltd, Nihon Kayaku, Eisai, Bayer Co., Ltd, Lilly, Taiho Pharmaceutical Co., Ltd, Daiichi Sankyo Co., Ltd, Yakult Honsha Co., Ltd, Nihon Servier, Asahi Kasei, and Ono Pharmaceutical Co., Ltd. Masahito Kotaka has received payment or honoraria for lectures, presentations, speaker’s bureau, manuscript writing, or educational events from Chugai Pharmaceutical Co. Ltd, Taiho Pharmaceutical Co., Ltd, and Yakult Honsha Co., Ltd. Toshiki Masuishi has received institutional grants or contracts from MSD, Daiichi Sankyo Co., Ltd, Ono Pharmaceutical Co., Ltd, and Novartis and personal consulting fees from Takeda Pharma, Chugai Pharmaceutical Co. Ltd, Merck Biopharma, Taiho Pharmaceutical Co., Ltd, Bayer, Eli Lilly Japan, Yakult Honsha Co., Ltd, Sanofi Co. Ltd, Daiichi Sankyo Co., Ltd, Ono Pharmaceutical Co., Ltd, and Bristol Myers Squibb. Hirokazu Shoji has received honoraria from Ono Pharmaceutical Co., Ltd and Zymeworks Inc.; has been on advisory boards for Personal Amgen, Astellas, Daiichi Sankyo Co., Ltd, MSD, and Takeda Pharma; and a local PI. Akitaka Makiyama has received honoraria from Eli Lilly Japan K.K., Taiho Pharmaceutical Co., Ltd, Ono Pharmaceutical Co., Ltd., Bristol-Myers Squibb Co. Ltd., and Daiichi Sankyo Co. Ltd. Naoki Takahashi has received honoraria from Ono Pharmaceutical Co, Ltd, Taiho Pharmaceutical Co. Ltd, and Bristol Myers Squibb. Takashi Ohta has received honoraria from Eli Lilly, Bristol-Myers Squibb K.K., Taiho Pharmaceutical Co. Ltd, Ono Pharmaceutical Co., Ltd, Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Takeda Pharmaceutical Co., Ltd., Yakult Honsha Co., Ltd., Daiichi Sankyo Co., Ltd., Otsuka Pharmaceutical Co., Ltd., EA Pharma Co., Ltd, and Merck & Co., Inc. Yosuke Kito has received honoraria from Taiho Pharmaceutical Co. Ltd, Ono Pharmaceutical Co., Ltd, and Daiichi Sankyo Co., Ltd. Narikazu Boku has received lecture fees or honoraria from Bristol-Myers Squibb, Daiichi-Sankyo Co., Ltd, Ono Pharmaceutical Co., Ltd, and Taiho Pharmaceutical Co. Ltd, and research funds from Ono Pharmaceutical Co., Ltd and Takeda. Kentaro Yamazaki has received honoraria for lectures from Chugai Pharmaceutical Co., Ltd., Yakult Honsha Co., Ltd., Daiichi Sankyo Co., Ltd., Merck Biopharma Co., Ltd., Sanofi K.K., MSD K.K., Takeda Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd., Eli Lilly Japan K.K, Ono Pharmaceutical Co., Ltd., and Bristol-Myers Squibb K.K. Shuichi Hironaka has received lecture fees or honoraria from Taiho Pharmaceutical Co., Ltd. Kei Muro has received lecture fees or honoraria from Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Bristol-Myers Squibb Co., Ltd., Eli Lilly Japan K.K., MSD K.K., Takeda Pharmaceutical Co., Ltd., Daiichi-Sankyo Co., Ltd, and Taiho Pharmaceutical Co. Ltd; research funds from Chugai Pharmaceutical Co., Ltd., MSD K.K., Amgen K.K., Ono Pharmaceutical Co., Ltd., Astellas Pharma Inc., Sanofi Co., Ltd., Taiho Pharmaceutical Co. Ltd., Eisai Co., Ltd., Daiichi-Sankyo Co. Ltd., Novartis Pharma K.K., and Pfizer Japan Inc., and has been an advisor for AstraZeneca K.K., Ono Pharmaceutical Co., Ltd., and Amgen K.K. Yasushi Tsuji, Kenro Hirata, Takao Tsuduki, Naoki Izawa, Masahiro Tsuda, Hisateru Yasui, Satoshi Otsu, Ichinosuke Hyodo, and Kenichi Yoshimura have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
This study was approved by the certified Review Board of Aichi Cancer Center Hospital and has been registered in the Japan Registry of Clinical Trials (jRCTs041190100).
Consent to Participate
Written informed consent was obtained from all patients before study enrollment.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Authors’ Contributions
Study concept—TM, KY, and KM. Study design—TM, KY and KM. Acquisition, analysis, or interpretation of data—All authors. Statistical analysis—NI and KY. Manuscript preparation—TM, NI, SH, NB and IH. Manuscript editing and revision—All authors. Manuscript review and approval—All authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Matsumoto, T., Yamamoto, Y., Kotaka, M. et al. A Phase II Study of FOLFIRI Plus Ziv-Aflibercept After Trifluridine/Tipiracil Plus Bevacizumab in Patients with Metastatic Colorectal Cancer: WJOG 11018G. Targ Oncol 19, 181–190 (2024). https://doi.org/10.1007/s11523-024-01043-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-024-01043-2