Abstract
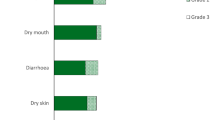
Pemigatinib (Pemazyre®), a selective, potent, reversible, oral inhibitor of fibroblast growth factor receptor (FGFR) 1–3, has received conditional (in the EU) or accelerated (in the USA) approval for the treatment of adults with previously treated, unresectable locally-advanced or metastatic cholangiocarcinoma (CCA) with an FGFR2 gene fusion or rearrangement. Over the course of a single-arm, phase 2 study (FIGHT-202), just over a third of patients with pretreated, advanced CCA [almost exclusively intrahepatic CCA (iCCA)] harbouring an FGFR2 fusion or rearrangement who received pemigatinib once daily (2 weeks on, 1 week off) had an objective response; nearly half had stable disease. Median progression-free survival and overall survival at the time of the final analysis were 7.0 months and 17.5 months, respectively. Pemigatinib was generally well tolerated and had a manageable safety profile. The most common treatment-related adverse event, hyperphosphataemia, was exclusively grade 1–2 in severity and, similarly, observed ocular and nail toxicities were rarely grade ≥ 3 in severity. Pending confirmation of its clinical benefits in an ongoing cisplatin plus gemcitabine-controlled, phase 3 study (FIGHT-302), pemigatinib provides a valuable targeted therapy for pretreated patients with advanced (i)CCA harbouring a FGFR2 fusion or rearrangement.
Plain Language Summary
Bile duct cancer or cholangiocarcinoma (CCA) has a very poor prognosis, partly because the majority of patients are first diagnosed at an advanced stage when they are no longer eligible for potentially curative surgery and are therefore limited to receiving systemic (palliative) chemotherapy, which results in only modest survival gains. 10–20% of CCAs arise inside the liver [intrahepatic CCAs (iCCAs)]; ≈ 10–20% of patients with advanced iCCAs are eligible to receive fibroblast growth factor receptors (FGFR) inhibitors, as the development of their tumours depends, in part, on FGFRs that have been inappropriately activated due to underlying genetic abnormalities. Pemigatinib (Pemazyre®) is a selective, potent, once-daily oral FGFR 1–3 inhibitor. In a phase 2 trial in patients with advanced CCA (almost exclusively iCCA) containing an abnormal FGFR2 fusion or rearrangement who had already received systemic chemotherapy, more than a third receiving pemigatinib experienced partial or complete shrinkage of their tumours, while almost half had neither growth nor shrinkage of their tumours. Pemigatinib was generally well tolerated with a manageable safety profile. Pending completion of a phase 3 study designed to confirm its clinical benefits, pemigatinib represents a valuable targeted therapy for pretreated patients with advanced (i)CCA harbouring a FGFR2 fusion or rearrangement.

Similar content being viewed by others
References
Vogel A, Bridgewater J, Edeline J, et al. Biliary tract cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up*. Ann Oncol. 2023;34(2):127–40.
Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557–88.
Javle M, Lee S, Azad NS, et al. Temporal changes in cholangiocarcinoma incidence and mortality in the United States from 2001 to 2017. Oncologist. 2022;27(10):874–83.
Saha SK, Zhu AX, Fuchs CS, et al. Forty-year trends in cholangiocarcinoma incidence in the US: intrahepatic disease on the rise. Oncologist. 2016;21:594–9.
Bertuccio P, Malvezzi M, Carioli G, et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019;71(1):104–14.
Florio AA, Ferlay J, Znaor A, et al. Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012. Cancer. 2020;126(11):2666–78.
Ioffe D, Phull P, Dotan E. Optimal mnagement of patients with advanced or metastatic cholangiocarcinoma: an evidence-based review. Cancer Manag Res. 2021;13:8085–98.
Bekaii-Saab TS, Valle JW, Van Cutsem E, et al. FIGHT-302: first-line pemigatinib vs gemcitabine plus cisplatin for advanced cholangiocarcinoma with FGFR2 rearrangements. Future Oncol. 2020;16(30):2385–99.
Harding JJ, Khalil DN, Fabris L, et al. Rational development of combination therapies for biliary tract cancers. J Hepatol. 2023;78(1):217–28.
Neumann O, Burn TC, Allgäuer M, et al. Genomic architecture of FGFR2 fusions in cholangiocarcinoma and its implication for molecular testing. Br J Cancer. 2022;127(8):1540–9.
Goyal L, Kongpetch S, Crolley VE, et al. Targeting FGFR inhibition in cholangiocarcinoma. Cancer Treat Rev. 2021;95: 102170.
Hoy SM. Pemigatinib: first approval. Drugs. 2020;80(9):923–9.
Incyte Biosciences Distribution B.V., Pemazyre 4.5 mg, 9 mg and 13.5 mg tablets : EU summary of product charcteristics. 2021. https://www.ema.europa.eu. Accessed 1 Nov 2023.
Incyte Corporation. PEMAZYRE™ (pemigatinib) tablets, for oral use: US prescribing information. 2022. https://www.pemazyre.com/pi/pi. Accessed 1 Nov 2023.
Incyte Corporation. Incyte announces approval of Pemazyre® (pemigatinib) in Japan for the treatment of patients with unresectable biliary tract cancer (BTC) with a fibroblast growth factor receptor 2 (FGFR2) fusion gene, worsening after cancer chemotherapy [media release]. 23 Mar 2021. http://www.incyte.com.
Incyte Corporation. Incyte announces Japanese approval of pemazyre® (Pemigatinib) for the treatment of patients with myeloid/lymphoid neoplasms (MLNs) [media release]. 27 Mar 2023. http://www.incyte.com.
Liu PCC, Koblish H, Wu L, et al. INCB054828 (pemigatinib), a potent and selective inhibitor of fibroblast growth factor receptors 1, 2, and 3, displays activity against genetically defined tumor models. PLoS ONE. 2020;15(4):1–16.
Rodon J, Damian S, Furqan M, et al. Clinical and translational findings of pemigatinib in previously treated solid tumors with activating FGFR1-3 alterations in the FIGHT-207 study [abstract no. CT016 plus poster]. Cancer Res. 2023;83(8 Suppl):CT016.
Kommalapati A, Tella SH, Borad M, et al. FGFR inhibitors in oncology: insight on the management of toxicities in clinical practice. Cancers (Basel). 2021;13(12):2968.
Ji T, Chen X, Liu X, et al. Population pharmacokinetics analysis of pemigatinib in patients with advanced malignancies. Clin Pharmacol Drug Dev. 2022;11(4):454–66.
Subbiah V, Iannotti NO, Gutierrez M, et al. FIGHT-101, a first-in-human study of potent and selective FGFR 1–3 inhibitor pemigatinib in pan-cancer patients with FGF/FGFR alterations and advanced malignancies. Ann Oncol. 2022;33(5):522–33.
Ji T, Rockich K, Epstein N, et al. Evaluation of the pharmacokinetics of pemigatinib in patients with impaired hepatic or renal function. Br J Clin Pharmacol. 2022;88(1):237–47.
Ji T, Rockich K, Epstein N, et al. Evaluation of drug-drug interactions of pemigatinib in healthy participants. Eur J Clin Pharmacol. 2021;77(12):1887–97.
Ji T, Chen X, Yeleswaram S. Evaluation of drug-drug interaction potential for pemigatinib using physiologically based pharmacokinetic modeling. CPT Pharmacometrics Syst Pharmacol. 2022;11(7):894–905.
Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21(5):671–84.
Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated locally advanced/metastatic cholangiocarcinoma (CCA): update of FIGHT-202 [abstract no. 4086 plus poster]. J Clin Oncol. 2021;39(15 Suppl):4086.
Vogel A, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated locally advanced or metastatic cholangiocarcinoma: final results from FIGHT-202 [abstract no. O-2 plus poster]. Ann Oncol. 2022;33(S4):S379.
Bibeau K, Féliz L, Lihou CF, et al. Progression-free survival in patients with cholangiocarcinoma with or without FGF/FGFR alterations: a FIGHT-202 post hoc analysis of prior systemic therapy response. JCO Precis Oncol. 2022;6:1–10.
Abou-Alfa G, Sahai V, Hollebecque A, et al. Progression-free survival in patients with cholangiocarcinoma with FGFR2 fusions or rearrangements: a FIGHT-202 post-hoc analysis of prior systemic therapy response [abstract no. SO-4 plus oral presentation]. Ann Oncol. 2021;32(Suppl 3):S203–4.
European Medicines Agency. Pemazyre (pemigatinib): assessment report. https://www.ema.europa.eu. Accessed 17 Nov 2023.
Silverman IM, Hollebecque A, Friboulet L, et al. Clinicogenomic analysis of FGFR2-rearranged cholangiocarcinoma identifies correlates of response and mechanisms of resistance to pemigatinib. Cancer Discov. 2021;11(2):326–39.
Shi G, Huang X, Wen T, et al. Pemigatinib in Chinese patients with advanced/metastatic or surgically unresectable cholangiocarcinoma Including FGFR2 fusion or rearrangement: updated overall survival from an open-label, single-arm, multicenter phase II study [abstract no. CT153]. Cancer Res. 2023;83(8 Suppl):319.
Shi GM, Huang XY, Wen TF, et al. Pemigatinib in previously treated Chinese patients with locally advanced or metastatic cholangiocarcinoma carrying FGFR2 fusions or rearrangements: a phase II study. Cancer Med. 2023;12(4):4137–46.
Subbiah V, Verstovsek S. Clinical development and management of adverse events associated with FGFR inhibitors. Cell Rep Med. 2023;4(10):101204.
NCCN clinical practice guidelines in oncology (NCCN guidelines®) biliary tract cancers version 2.2023—May 10, 2023. https://www.nccn.org. Accessed 1 Nov 2023.
Normanno N, Martinelli E, Melisi D, et al. Role of molecular genetics in the clinical management of cholangiocarcinoma. ESMO Open. 2022;7(3):100505.
Angerilli V, Fornaro L, Pepe F, et al. FGFR2 testing in cholangiocarcinoma: translating molecular studies into clinical practice. Pathologica. 2023;115(2):71–82.
Paine A, Thom H, Wacheck V, et al. Matching-adjusted indirect comparison of futibatinib versus chemotherapy and pemigatinib in cholangiocarcinoma patients with FGFR2 fusions/rearrangements [abstract no. CO78 plus poster]. Value Health. 2022;25(12 Suppl):S33.
Lindley A, Prager G, Bitzer M, et al. Global expanded access program (EAP) to pemigatinib for patients (pts) with previously treated locally advanced or metastatic cholangiocarcinoma (CCA) and FGFR2 fusions or rearrangements [abstract no. 335]. Oncol Res Treat. 2022;45(Suppl 3):81.
National Institute for Health Care Excellence. Pemigatinib for treating relapsed or refractory advanced cholangiocarcinoma with FGFR2 fusion or rearrangement. Technology appraisal guidance [TA722]. https://www.nice.org.uk. Accessed 1 Nov 2023.
Scottish Medicines Consortium. SMC2399. Pemigatinib 4. 5mg, 9 mg, and 13. 5mg tablets (Pemazyre®). https://www.scottishmedicines.org.uk. Accessed 17 Nov 2023.
Acknowledgements
During the peer review process, the manufacturer of pemigatinib was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
James E. Frampton is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
The manuscript was reviewed by: C. Hierro, Medical Oncology Department. Catalan Institute of Oncology-Badalona, Badalona-Applied Research Group in Oncology (B-ARGO), Badalona, Barcelona, Spain; M. A. Morse, Department of Medicine, Duke University Medical Center, Durham, North Carolina, USA.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Frampton, J.E. Pemigatinib: A Review in Advanced Cholangiocarcinoma. Targ Oncol 19, 107–114 (2024). https://doi.org/10.1007/s11523-023-01024-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-023-01024-x