Abstract
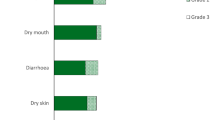
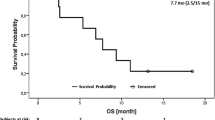
Ivosidenib (Tibsovo®), a first-in-class, oral small molecule, potent and selective inhibitor of mutant isocitrate dehydrogenase 1 (mIDH1), is approved in the EU and USA for the treatment of adults with pretreated, advanced, mIDH1 cholangiocarcinoma (CCA). It is presumed to exert its cytostatic effects in this setting by suppressing 2-hydroxyglutarate, an oncometabolite produced by mIDH1 that impairs cellular differentiation and promotes tumorigenesis. In the multinational phase 3 ClarIDHy study in patients with pretreated, advanced mIDH1 CCA, monotherapy with ivosidenib once daily significantly prolonged progression-free survival (PFS) and almost doubled the disease control rate compared with placebo. Moreover, it had a favourable effect on overall survival (OS), which was also significantly prolonged after correcting for a high rate of crossover from the placebo group (permitted by the trial protocol). Ivosidenib treatment preserved health-related quality of life (HRQOL) relating to physical function, pain and appetite loss/eating and was generally well tolerated, with the most common treatment-emergent adverse events being low-grade diarrhoea, nausea and fatigue. Thus, ivosidenib represents a novel and valuable targeted therapy for the subset of patients with pretreated, advanced CCA tumors harbouring mIDH1.
Plain Language Summary
Cholangiocarcinoma (CCA) is often diagnosed at an advanced stage when the prognosis is poor due to limited treatment options, including standard-of-care (palliative) chemotherapy. Around 40% of patients with CCA have a tumor that can be targeted for treatment due to the presence of a molecular abnormality implicated in tumor formation and/or maintenance. One such abnormality (present in ≈14% of patients with intrahepatic CCA), is a mutated form of the isocitrate dehydrogenase 1 (IDH1) enzyme (‘mutant IDH1’) that inappropriately catalyses the production of 2-hydroxyglutarate (2-HG), an oncometabolite that promotes tumorigenesis. Ivosidenib (Tibsovo®), a first-in-class, small molecule, oral inhibitor of mIDH1, exerts a cytostatic (stabilizing) effect on mIDH1 CCA tumors, presumably by suppressing the production of 2-HG. Compared with placebo, ivosidenib resulted in a statistically significant, near-doubling of progression-free survival and, similarly, a near doubling of the disease control rate in a pivotal study in patients with pretreated, advanced, mIDH1 CCA. Overall survival was also significantly improved after controlling for the high rate of crossover. Health-related quality of life was maintained and ivosidenib treatment was generally well tolerated. Ivosidenib represents a novel and valuable targeted therapy for patients with advanced mIDH1 CCA tumors.

Similar content being viewed by others
References
Vogel A, Bridgewater J, Edeline J, et al. Biliary tract cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(2):127–40.
Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Gastroenterol Hepatol. 2020;17:557–88.
Rimini M, Puzzoni M, Pedica F, et al. Cholangiocarcinoma: new perspectives for new horizons. Expert Rev Gastroenterol Hepatol. 2021;15(12):1367–83.
Lee Y-P, Oh SY, Kim KM, et al. Modified FOLFIRINOX as a second-line treatment for patients with gemcitabine-failed advanced biliary tract cancer: a prospective multicenter phase II study. Cancers (Basel). 2022;14(8):1950.
Adeva J, Sangro B, Salati M, et al. Medical treatment for cholangiocarcinoma. Liver Int. 2019;39(Suppl. 1):123–42.
Cheng C-Y, Chen C-P, Wu C-E. Precision medicine in cholangiocarcinoma: past, present and future. Life. 2022;12(6):829.
Boscoe AN, Rolland C, Kelley RK. Frequency and prognostic significance of isocitratdehydrogenase 1 mutations in cholangiocarcinoma: a systematic literature review. J Gastrointest Oncol. 2019;10(4):751–65.
Kendre G, Murugesan K, Brummer T, et al. Charting co-mutation patterns associated with actionable drivers in intrahepatic cholangiocarcinoma. J Hepatol. 2023;78(3):614–26.
Makawita S, Borad MJ, Carapeto F, et al. IDH1 and IDH2 driven intrahepatic cholangiocarcinoma (IHCC): a comprehensive genomic and immune profiling study [abstract no. 4009]. J Clin Oncol. 2021;39(15 suppl):4009.
Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462(7274):739.
Wu M-J, Shi L, Merritt J, et al. Biology of IDH mutant cholangiocarcinoma. Hepatology. 2022;75(5):1322–7.
Wu M-J, Shi L, Dubrot J, et al. Mutant IDH inhibits IFNγ-TET2 sgnaling to promote immunoevasion and tumor maintenance in cholangiocarcinoma. Cancer Discov. 2022;12(3):812–35.
Notarangelo G, Spinelli JB, Perez EM, et al. Oncometabolite d-2HG alters T cell metabolism to impair CD8(+) T cell function. Science. 2022;377:1519–29.
Saatcioglu HD, Valle J, Macarulla T, et al. Characteristics of the tumor microenvironment in IDH1-mutated cholangiocarcinoma patients from ClarIDHy trial [abstract no. 552]. J Immunother Cancer. 2022;10(Suppl 2):A576–7.
Saha SK, Parachoniak CA, Ghanta KS, et al. Mutant IDH inhibits HNF-4α to block hepatocyte differentiation and promote biliary cancer. Nature. 2014;513(7516):110–4.
Popovici-Muller J, Lemieux RM, Artin E, et al. Discovery of AG-120 (ivosidenib): a first-in-class mutant IDH1 inhibitor for the treatment of IDH1 mutant cancers. ACS Med Chem Lett. 2018;9(4):300–5.
Les Laboratoires Servier. Tibsovo (ivosidenib): EU prescribing information. 2023. https://www.ema.europa.eu/. Accessed 30 Sept 2023.
Servier Pharmaceutical LLC. TIBSOVO (ivosidenib tablets), for oral use. 2022. https://www.accessdata.fda.gov/. Accessed 30 Sept 2023.
Lowery MA, Burris HA 3rd, Janku F, et al. Safety and activity of ivosidenib in patients with IDH1-mutant advanced cholangiocarcinoma: a phase 1 study. Lancet Gastroenterol Hepatol. 2019;4(9):711–20.
Abou-Alfa GK, Macarulla T, Javle MM, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(6):796–807.
Aguado-Fraile E, Tassinari A, Ishii Y, et al. Molecular and morphological changes induced by ivosidenib correlate with efficacy in mutant-IDH1 cholangiocarcinoma. Future Oncol. 2021;17(16):2057–74.
European Medicines Agency. Tibsovo assessment report. 2023. https://www.ema.europa.eu/. Accessed 30 Sept 2023.
Dai D, Yang H, Nabhan S, et al. Effect of itraconazole, food, and ethnic origin on the pharmacokinetics of ivosidenib in healthy subjects. Eur J Clin Pharmacol. 2019;75(8):1099–108.
Prakash C, Fan B, Altaf S, et al. Pharmacokinetics, absorption, metabolism, and excretion of [14C] ivosidenib (AG-120) in healthy male subjects. Cancer Chemother Pharmacol. 2019;83(5):837–48.
Fan B, Dai D, Cohen M, et al. Effect of mild and moderate hepatic impairment on the pharmacokinetics, safety, and tolerability of a single dose of oral ivosidenib in otherwise healthy participants. Clin Pharmacol Drug Dev. 2021;10(1):99–109.
Zhu AX, Macarulla T, Javle MM, et al. Final overall survival efficacy results of ivosidenib for patients with advanced cholangiocarcinoma with IDH1 mutation: the phase 3 randomized clinical ClarIDHy trial. JAMA Oncol. 2021;7(11):1669–77.
Zhu AX, Macarulla T, Javle MM, et al. Final results from ClarIDHy, a global, phase III, randomized, double-blind study of ivosidenib (IVO) versus placebo (PBO) in patients (pts) with previously treated cholangiocarcinoma (CCA) and an isocitrate dehydrogenase 1 (IDH1) mutation [abstract no. 266 plus presentation #GI21]. J Clin Oncol. 2021;39(3 Suppl.):266.
Chamberlain CX, Hua Z, Gliser C, et al. Longitudinal trends in health-related quality of life (HRQoL) among patients treated with ivosidenib (IVO) for IDH1-mutated cholangiocarcinoma (CCA) in the ClarIDHy study [abstract no 388 plus poster]. J Clin Oncol. 2022;40(4 Suppl.):388.
Abou-Alfa GK, Burris S, Adeva J, et al. Characterization of IDH-1 mutant cholangiocarcinoma patients who received ivosidenib treatment longer than a year [poster]. In: 10th Annual cholangiocarcinoma foundation conference, 2023.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Biliary Tract Cancers Version 2.2023—May 10, 2023. https://www.nccn.org/. Accessed 30 Sept 2023.
Rimini M, Burgio V, Antonuzzo L, et al. Real-world data on ivosidenib in patients with previously treated isocitrate dehydrogenase 1-mutated intrahepatic cholangiocarcinomas: an early exploratory analysis. Target Oncol. 2022;17(5):591–6.
Reinbold R, Hvinden IC, Rabe P, et al. Resistance to the isocitrate dehydrogenase 1 mutant inhibitor ivosidenib can be overcome by alternative dimer-interface binding inhibitors. Nat Commun. 2022;13(1):4785.
Acknowledgments
During the peer review process, the manufacturer of ivosidenib was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of Interest
James E. Frampton is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics Approval, Consent to Participate, Consent to Publish, Availability of Data and Material, Code Availability
Not applicable.
Additional information
The manuscript was reviewed by: C. Hierro, Medical Oncology Department. Catalan Institute of Oncology-Badalona, Badalona-Applied Research Group in Oncology (B-ARGO), Badalona, Barcelona, Spain; A. Manne, Department of Internal Medicine, Division of Medical Oncology, The Ohio State University Wexner Medical Center, Columbus, OH, USA.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Frampton, J.E. Ivosidenib: A Review in Advanced Cholangiocarcinoma. Targ Oncol 18, 973–980 (2023). https://doi.org/10.1007/s11523-023-01002-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-023-01002-3