Abstract
Background
Osimertinib monotherapy is a common treatment for epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancer (NSCLC); however, standard treatment strategies for acquired resistance to this drug have not been established. In addition, the clinical significance of first-generation (1G) or second-generation (2G) EGFR-tyrosine kinase inhibitors (TKI) in patients with EGFR-mutant NSCLC and osimertinib resistance has not yet been fully evaluated.
Objective
We aimed to conduct a prospective multicenter observational study to evaluate the efficacy and safety of 1G and 2G EGFR-TKIs after the development of osimertinib resistance.
Methods
Patients with EGFR-mutant NSCLC who received 1G or 2G EGFR-TKIs after developing resistance to osimertinib monotherapy were prospectively assessed at eight institutions in Japan. The primary endpoint was progression-free survival (PFS).
Results
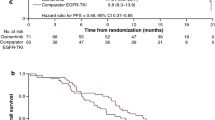
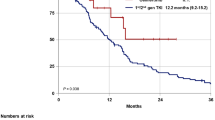
A total of 29 patients with advanced or recurrent EGFR-mutant NSCLC were analyzed. The objective response and disease control rates were 6.9% (2/29) and 58.6% (17/29), respectively. The median PFS was 1.9 months [95% confidence interval (CI): 1.3–5.3]. There was no significant difference in PFS between the 1G and 2G EGFR-TKI groups (3.7 versus 1.5 months, log-rank test p = 0.20). However, patients with normal cytokeratin 19 fragment (CYFRA 21-1) and pro-gastrin-releasing peptide (ProGRP) levels experienced longer PFS than those with elevated CYFRA 21-1 and/or ProGRP (5.5 versus 1.3 months, log-rank test p < 0.001).
Conclusion
Administration of 1G or 2G EGFR-TKIs after the development of osimertinib resistance has limited efficacy in patients with EGFR-mutant NSCLC. Moreover, normal CYFRA 21-1 and ProGRP levels could be promising indicators for 1G and 2G EGFR-TKI administration after osimertinib resistance development.
Trial Registration Number
UMIN000044049.


Similar content being viewed by others
References
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. https://doi.org/10.1056/NEJMoa0909530.
Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8. https://doi.org/10.1016/S1470-2045(09)70364-X.
Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–34. https://doi.org/10.1200/JCO.2012.44.2806.
Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:1454–66. https://doi.org/10.1016/S1470-2045(17)30608-3.
Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–25. https://doi.org/10.1056/NEJMoa1713137.
Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41–50. https://doi.org/10.1056/NEJMoa1913662.
Morimoto K, Sawada R, Yamada T, Azuma K, Ito K, Goto Y, et al. A real-world analysis of immune checkpoint inhibitor-based therapy after osimertinib treatment in patients with EGFR-mutant NSCLC. JTO Clin Res Rep. 2022;3:100388. https://doi.org/10.1016/j.jtocrr.2022.100388.
Morimoto K, Yamada T, Sawada R, Azuma K, Goto Y, Harada T, et al. Predictive value of p53 and AXL immunostaining for the efficacy of immune checkpoint inhibitor-based therapy after osimertinib treatment in patients with epidermal growth factor-mutant non-small cell lung cancer. Cancer Immunol Immunother. 2023;72:1699–707. https://doi.org/10.1007/s00262-023-03370-1.
Song Y, Wu YL, Cao LJ, Chen JH, Ma ZY, Cui JW, et al. Efficacy and safety of gefitinib as third-line treatment in NSCLC patients with activating EGFR mutations treated with first-line gefitinib followed by second-line chemotherapy:a single-arm, prospective, multicenter phase II study (RE-CHALLENGE, CTONG1304). Am J Clin Oncol. 2019;42:432–9. https://doi.org/10.1097/COC.0000000000000538.
Yamaguchi O, Kaira K, Mouri A, Shiono A, Hashimoto K, Miura Y, et al. Re-challenge of afatinib after 1st generation EGFR-TKI failure in patients with previously treated non-small cell lung cancer harboring EGFR mutation. Cancer Chemother Pharmacol. 2019;83:817–25. https://doi.org/10.1007/s00280-019-03790-w.
Oda N, Hotta K, Ninomiya K, Minami D, Ichihara E, Murakami T, et al. A phase II trial of EGFR-TKI readministration with afatinib in advanced non-small-cell lung cancer harboring a sensitive non-T790M EGFR mutation: Okayama Lung Cancer Study Group trial 1403. Cancer Chemother Pharmacol. 2018;82:1031–8. https://doi.org/10.1007/s00280-018-3694-5.
Chang GC, Tseng CH, Hsu KH, Yu CJ, Yang CT, Chen KC, et al. Predictive factors for EGFR-tyrosine kinase inhibitor retreatment in patients with EGFR-mutated non-small-cell lung cancer—a multicenter retrospective SEQUENCE study. Lung Cancer. 2017;104:58–64. https://doi.org/10.1016/j.lungcan.2016.12.002.
Ichihara E, Hotta K, Ninomiya K, Kubo T, Ohashi K, Rai K, et al. Re-administration of osimertinib in osimertinib-acquired resistant non-small-cell lung cancer. Lung Cancer. 2019;132:54–8. https://doi.org/10.1016/j.lungcan.2019.02.021.
Fu K, Xie F, Wang F, Fu L. Therapeutic strategies for EGFR-mutated non-small cell lung cancer patients with osimertinib resistance. J Hematol Oncol. 2022;15:173. https://doi.org/10.1186/s13045-022-01391-4.
Ercan D, Choi HG, Yun CH, Capelletti M, Xie T, Eck MJ, et al. EGFR mutations and resistance to irreversible pyrimidine-based EGFR inhibitors. Clin Cancer Res. 2015;21:3913–23. https://doi.org/10.1158/1078-0432.CCR-14-2789.
Fassunke J, Müller F, Keul M, Michels S, Dammert MA, Schmitt A, et al. Overcoming EGFRG724S-mediated osimertinib resistance through unique binding characteristics of second-generation EGFR inhibitors. Nat Commun. 2018;9:4655. https://doi.org/10.1038/s41467-018-07078-0.
Brown BP, Zhang YK, Westover D, Yan Y, Qiao H, Huang V, et al. On-target resistance to the mutant-selective EGFR inhibitor osimertinib can develop in an allele-specific manner dependent on the original EGFR-activating mutation. Clin Cancer Res. 2019;25:3341–51. https://doi.org/10.1158/1078-0432.CCR-18-3829.
Yoshimura A, Yamada T, Okuma Y, Fukuda A, Watanabe S, Nishioka N, et al. Impact of tumor programmed death ligand-1 expression on osimertinib efficacy in untreated EGFR-mutated advanced non-small cell lung cancer: a prospective observational study. Transl Lung Cancer Res. 2021;10:3582–93. https://doi.org/10.21037/tlcr-21-461.
Sakata Y, Sakata S, Oya Y, Tamiya M, Suzuki H, Shibaki R, et al. Osimertinib as first-line treatment for advanced epidermal growth factor receptor mutation-positive non-small-cell lung cancer in a real-world setting (OSI-FACT). Eur J Cancer. 2021;159:144–53. https://doi.org/10.1016/j.ejca.2021.09.041.
Nakamura H, Nishimura T. History, molecular features, and clinical importance of conventional serum biomarkers in lung cancer. Surg Today. 2017;47:1037–59. https://doi.org/10.1007/s00595-017-1477-y.
Yoshimura A, Uchino J, Hasegawa K, Tsuji T, Shiotsu S, Yuba T, et al. Carcinoembryonic antigen and CYFRA 21–1 responses as prognostic factors in advanced non-small cell lung cancer. Transl Lung Cancer Res. 2019;8:227–34. https://doi.org/10.21037/tlcr.2019.06.08.
Takeuchi A, Oguri T, Sone K, Ito K, Kitamura Y, Inoue Y, et al. Predictive and prognostic value of CYFRA 21–1 for advanced non-small cell lung cancer treated with EGFR-TKIs. Anticancer Res. 2017;37:5771–6. https://doi.org/10.21873/anticanres.12018.
Feng LX, Wang J, Yu Z, Song SA, Zhai WX, Dong SH, et al. Clinical significance of serum EGFR gene mutation and serum tumor markers in predicting tyrosine kinase inhibitor efficacy in lung adenocarcinoma. Clin Transl Oncol. 2019;21:1005–13. https://doi.org/10.1007/s12094-018-02014-6.
Dong J, Tong S, Shi X, Wang C, Xiao X, Ji W, et al. Progastrin-releasing peptide precursor and neuron-specific enolase predict the efficacy of first-line treatment with epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors among non-small-cell lung cancer patients harboring EGFR mutations. Cancer Manag Res. 2020;12:13607–16. https://doi.org/10.2147/CMAR.S285121.
Oh HJ, Park HY, Kim KH, Park CK, Shin HJ, Lim JH, et al. Progastrin-releasing peptide as a diagnostic and therapeutic biomarker of small cell lung cancer. J Thorac Dis. 2016;8:2530–7. https://doi.org/10.21037/jtd.2016.08.72.
Suh KJ, Keam B, Kim M, Park YS, Kim TM, Jeon YK, et al. Serum neuron-specific enolase levels predict the efficacy of first-line epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in patients with non-small cell lung cancer harboring EGFR mutations. Clin Lung Cancer. 2016;17:245-252.e1. https://doi.org/10.1016/j.cllc.2015.11.012.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8. https://doi.org/10.1038/bmt.2012.244.
Choudhury NJ, Makhnin A, Tobi YY, Daly RM, Preeshagul IR, Iqbal AN, et al. Pilot study of dacomitinib for patients with metastatic EGFR-mutant lung cancers with disease progression after initial treatment with osimertinib. JCO Precis Oncol. 2021;5:PO.21.00005. https://doi.org/10.1200/PO.21.00005.
Aredo JV, Wakelee HA, Neal JW, Padda SK. Afatinib after progression on osimertinib in EGFR-mutated non-small cell lung cancer. Cancer Treat Res Commun. 2021;30:100497. https://doi.org/10.1016/j.ctarc.2021.100497
Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121:725–37. https://doi.org/10.1038/s41416-019-0573-8.
Choudhury NJ, Marra A, Sui JSY, Flynn J, Yang SR, Falcon CJ, et al. Molecular biomarkers of disease outcomes and mechanisms of acquired resistance to first-line osimertinib in advanced EGFR-mutant lung cancers. J Thorac Oncol. 2023;18:463–75. https://doi.org/10.1016/j.jtho.2022.11.022.
Schoenfeld AJ, Chan JM, Kubota D, Sato H, Rizvi H, Daneshbod Y, et al. Tumor analyses reveal squamous transformation and off-target alterations as early resistance mechanisms to first-line osimertinib in EGFR-mutant lung cancer. Clin Cancer Res. 2020;26:2654–63. https://doi.org/10.1158/1078-0432.CCR-19-3563.
Yoshimura A, Yamada T, Serizawa M, Uehara H, Tanimura K, Okuma Y, et al. High levels of AXL expression in untreated EGFR-mutated non-small cell lung cancer negatively impacts the use of osimertinib. Cancer Sci. 2023;114:606–18. https://doi.org/10.1111/cas.15608.
Tanaka K, Hata A, Kaji R, Fujita S, Otoshi T, Fujimoto D, et al. Cytokeratin 19 fragment predicts the efficacy of epidermal growth factor receptor-tyrosine kinase inhibitor in non-small-cell lung cancer harboring EGFR mutation. J Thorac Oncol. 2013;8:892–8. https://doi.org/10.1097/JTO.0b013e31828c3929.
Shibayama T, Ueoka H, Nishii K, Kiura K, Tabata M, Miyatake K, et al. Complementary roles of pro-gastrin-releasing peptide (ProGRP) and neuron specific enolase (NSE) in diagnosis and prognosis of small-cell lung cancer (SCLC). Lung Cancer. 2001;32:61–9. https://doi.org/10.1016/s0169-5002(00)00205-1.
Watanabe S, Sone T, Matsui T, Yamamura K, Tani M, Okazaki A, et al. Transformation to small-cell lung cancer following treatment with EGFR tyrosine kinase inhibitors in a patient with lung adenocarcinoma. Lung Cancer. 2013;82:370–2. https://doi.org/10.1016/j.lungcan.2013.06.003.
Kato Y, Tanaka Y, Hino M, Gemma A. ProGRP as early predictive marker of non-small-cell lung cancer to small-cell lung cancer transformation after EGFR-TKI treatment. Respir Med Case Rep. 2019;27:100837. https://doi.org/10.1016/j.rmcr.2019.100837.
Ogusu S, Ariyasu R, Akita T, Kiritani A, Tsugitomi R, Amino Y, et al. EGFR-TKI re-administration after osimertinib failure in T790M mutation loss cases with re-biopsy. Invest New Drugs. 2022;40:1342–9. https://doi.org/10.1007/s10637-022-01301-y.
Inoue A, Kobayashi K, Usui K, Maemondo M, Okinaga S, Mikami I, et al. First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol. 2009;27:1394–400. https://doi.org/10.1200/JCO.2008.18.7658.
Yang Z, Hackshaw A, Feng Q, Fu X, Zhang Y, Mao C, et al. Comparison of gefitinib, erlotinib and afatinib in non-small cell lung cancer: a meta-analysis. Int J Cancer. 2017;140:2805–19. https://doi.org/10.1002/ijc.30691.
Araki T, Kanda S, Komatsu M, Sonehara K, Tateishi K, Takada M, et al. Rechallenge of afatinib for EGFR-mutated non-small cell lung cancer previously treated with osimertinib: a multicenter phase II trial protocol (REAL study). Transl Lung Cancer Res. 2023;12:1320–7. https://doi.org/10.21037/tlcr-23-12.
Acknowledgments
The authors sincerely appreciate the contributions of all the physicians and patients who participated in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflicts of Interest
Tadaaki Yamada received grants from Pfizer, Ono Pharmaceutical, Janssen Pharmaceutical, AstraZeneca, and Takeda Pharmaceutical as well as personal fees from Eli Lilly. Koichi Takayama received grants from Chugai Pharmaceutical and Ono Pharmaceutical as well as personal fees from AstraZeneca, Chugai Pharmaceutical, MSD, Eli Lilly, Boehringer Ingelheim, and Daiichi Sankyo. Kenji Morimoto, Takayuki Takeda, Shinsuke Shiotsu, Koji Date, Nobuyo Tamiya, Yasuhiro Goto, Hibiki Kanda, Yusuke Chihara, Yusuke Kunimatsu, Yuki Katayama, Masahiro Iwasaku, and Shinsaku Tokuda declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Availability of Data and Material
The datasets generated during the current study are not publicly available because of ethical constraints. However, they are available from the corresponding author upon reasonable request.
Ethics Approval
This study was approved by the Institutional Review Board of Kyoto Prefectural University of Medicine (ERB-C- 1731) and each participating hospital, registered at the University Medical Hospital Information Network (UMIN) Clinical Trials Registry (UMIN000044049), and performed in accordance with the Declaration of Helsinki.
Consent to Participate
All patients provided written informed consent prior to participation in this prospective study. In addition, opt-out informed consent was provided at each hospital where the trial was conducted.
Code Availability
Not applicable.
Author Contributions
TY was responsible for conceptualization, methodology, formal analysis, writing-original draft preparation, writing-review and editing, and supervision, KM for methodology, investigation, formal analysis, writing-original draft preparation, writing-review and editing, project administration, and data curation; TT for methodology; Shinsuke Shiotsu for investigation; Koji Date for investigation; Nobuyo Tamiya for investigation; YG for investigation; Hibiki Kanda for investigation; YC for investigation; YK for investigation; YK for project administration and data curation; MI for project administration and data curation; ST for project administration and data curation; and KT for writing-original draft preparation, writing-review and editing, and supervision.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Morimoto, K., Yamada, T., Takeda, T. et al. Clinical Efficacy and Safety of First- or Second-Generation EGFR-TKIs after Osimertinib Resistance for EGFR Mutated Lung Cancer: A Prospective Exploratory Study. Targ Oncol 18, 657–665 (2023). https://doi.org/10.1007/s11523-023-00991-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-023-00991-5