Abstract
Background
Epidermal growth factor receptor (EGFR)- and human epidermal growth factor receptor (HER)2-targeted therapies are approved for the treatment of breast, gastric/gastrointestinal junction (GEJ), and non-small cell lung cancer (NSCLC) with specific molecular aberrations affecting HER family members. Over 10 % of other cancers harbor genomic aberrations affecting HER family members, but their role remains undefined.
Objective
The MOBILITY3 trial evaluated the antitumor activity of afatinib, an oral pan-HER tyrosine kinase inhibitor (TKI) in HER-aberrant tumors outside of the licensed indications.
Patients and Methods
In this single-center basket trial, patients with advanced solid tumors that harbor mutations and/or amplifications of any of the HER family members (EGFR, ERBB2, ERBB3, ERBB4) were enrolled. The EGFR-mutated NSCLC and HER2-positive breast cancers were excluded. Participants were treated with oral afatinib 40 mg daily until disease progression or unacceptable toxicity. Objective response rate (ORR) and progression-free survival (PFS) were primary and secondary endpoints, respectively.
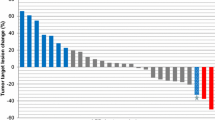
Results
The study enrolled 12 patients with 6 tumor types (NSCLC, sarcoma, salivary gland, gastric/GEJ, breast and pancreatic cancer). Objective response rate was 8 % (95 % CI 0.2–38%) and median PFS was 11.4 weeks (95% CI 4.6–33.3 weeks). All 3 patients with salivary gland cancers and 1 patient with ERBB2-mutant NSCLC had clinical benefit (stable disease or partial response lasting > 24 weeks). Due to slow accrual and a lower-than-expected response rate, trial recruitment was terminated before the target of 30 patients were enrolled.
Conclusions
In the MOBILITY3 study (NCT02506517), afatinib demonstrated modest activity in tumors that possess EGFR and ERBB2 aberrations. Clinical benefit seen in all 3 salivary gland cancers supports the growing evidence for the utility of HER-targeted therapies in the treatment of this specific tumor type.


Similar content being viewed by others
References
Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer. 2012;12(8):553–63.
Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res. 2006;12(18):5268–72.
Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154–62.
Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958–67.
Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–500.
Oh DY, Bang YJ. HER2-targeted therapies—a role beyond breast cancer. Nat Rev Clin Oncol. 2020;17(1):33–48.
Nelson V, Ziehr J, Agulnik M, Johnson M. Afatinib: emerging next-generation tyrosine kinase inhibitor for NSCLC. Onco Targets Ther. 2013;6:135–43.
Li D, Ambrogio L, Shimamura T, Kubo S, Takahashi M, Chirieac LR, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27(34):4702–11.
Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–34.
Stockley TL, Oza AM, Berman HK, Leighl NB, Knox JJ, Shepherd FA, et al. Molecular profiling of advanced solid tumors and patient outcomes with genotype-matched clinical trials: the Princess Margaret IMPACT/COMPACT trial. Genome Med. 2016;8(1):109.
Harbeck N, Im S-A, Huang C-S, Im Y-H, Xu B, Hurvitz SA, et al. LUX-breast 1: Randomized, phase III trial of afatinib and vinorelbine versus trastuzumab and vinorelbine in patients with HER2-overexpressing metastatic breast cancer (MBC) failing one prior trastuzumab treatment. J Clin Oncol. 2012;30(15_suppl):TPS649-TPS.
Sukhai MA, Craddock KJ, Thomas M, Hansen AR, Zhang T, Siu L, et al. A classification system for clinical relevance of somatic variants identified in molecular profiling of cancer. Genet Med. 2016;18(2):128–36.
Pugh TJ, Amr SS, Bowser MJ, Gowrisankar S, Hynes E, Mahanta LM, et al. VisCap: inference and visualization of germ-line copy-number variants from targeted clinical sequencing data. Genet Med. 2016;18(7):712–9.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Bedard PL, Oza AM, Tsao M-S, Leighl NB, Shepherd FA, Chen EX, et al. Princess Margaret Cancer Centre (PMCC) Integrated Molecular Profiling in Advanced Cancers Trial (IMPACT) using genotyping and targeted next-generation sequencing (NGS). J Clin Oncol. 2013;31(15):11002.
Ferté C, Massard C, Ileana E, Hollebecque A, Lacroix L, Ammari S, et al. Abstract CT240: Molecular screening for cancer treatment optimization (MOSCATO 01): a prospective molecular triage trial; Interim analysis of 420 patients. Cancer Research. 2014;74(19 Supplement):CT240-CT.
Tanaka H, Taima K, Itoga M, Ishioka Y, Baba K, Shiratori T, et al. Real-world study of afatinib in first-line or re-challenge settings for patients with EGFR mutant non-small cell lung cancer. Med Oncol. 2019;36(6):57.
Halmos B, Tan EH, Soo RA, Cadranel J, Lee MK, Foucher P, et al. Impact of afatinib dose modification on safety and effectiveness in patients with EGFR mutation-positive advanced NSCLC: results from a global real-world study (RealGiDo). Lung Cancer. 2019;127:103–11.
Hyman DM, Piha-Paul SA, Won H, Rodon J, Saura C, Shapiro GI, et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature. 2018;554(7691):189–94.
Oaknin A, Friedman CF, Roman LD, D’Souza A, Brana I, Bidard FC, et al. Neratinib in patients with HER2-mutant, metastatic cervical cancer: Findings from the phase 2 SUMMIT basket trial. Gynecol Oncol. 2020;159(1):150–6.
Harding JJ, Cleary JM, Quinn DI, Braña I, Moreno V, Borad MJ, et al. Targeting HER2 (ERBB2) mutation-positive advanced biliary tract cancers with neratinib: results from the phase II SUMMIT ‘basket’ trial. J Clin Oncol. 2021;39(3):320.
Kris MG, Camidge DR, Giaccone G, Hida T, Li BT, O’Connell J, et al. Targeting HER2 aberrations as actionable drivers in lung cancers: phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amplified tumors. Ann Oncol. 2015;26(7):1421–7.
Dziadziuszko R, Smit EF, Dafni U, Wolf J, Wasag B, Biernat W, et al. Afatinib in NSCLC With HER2 Mutations: Results of the Prospective, Open-Label Phase II NICHE Trial of European Thoracic Oncology Platform (ETOP). J Thorac Oncol. 2019;14(6):1086–94.
Zhou C, Li X, Wang Q, Gao G, Zhang Y, Chen J, et al. Pyrotinib in HER2-mutant advanced lung adenocarcinoma after platinum-based chemotherapy: a multicenter, open-label, single-arm, Phase II Study. J Clin Oncol. 2020;38(24):2753–61.
Robichaux JP, Elamin YY, Vijayan RSK, Nilsson MB, Hu L, He J, et al. Pan-Cancer Landscape and Analysis of ERBB2 Mutations Identifies Poziotinib as a Clinically Active Inhibitor and Enhancer of T-DM1 Activity. Cancer Cell. 2019;36(4):444-57e7.
Elamin YY, Robichaux JP, Carter BW, Altan M, Gibbons DL, Fossella FV, et al. Poziotinib for patients with HER2 exon 20 mutant non-small-cell lung cancer: results from a phase II trial. J Clin Oncol. 2022;40(7):702–9.
Cornelissen R, Sun S, Wollner M, Garassino MCC, Prelaj A, Haura EB, et al. LBA46 Efficacy and safety of poziotinib in treatment-naïve NSCLC harboring HER2 exon 20 mutations: a multinational phase II study (ZENITH20-4). Ann Oncol. 2021;32:S1324.
Kurzrock R, Bowles DW, Kang H, Meric-Bernstam F, Hainsworth J, Spigel DR, et al. Targeted therapy for advanced salivary gland carcinoma based on molecular profiling: results from MyPathway, a phase IIa multiple basket study. Ann Oncol. 2020;31(3):412–21.
Li BT, Makker V, Buonocore DJ, Offin MD, Olah ZT, Panora E, et al. A multi-histology basket trial of ado-trastuzumab emtansine in patients with HER2 amplified cancers. J Clin Oncol. 2018;36(15):2502.
Li BT, Shen R, Offin M, Buonocore DJ, Myers ML, Venkatesh A, et al. Ado-trastuzumab emtansine in patients with HER2 amplified salivary gland cancers (SGCs): Results from a phase II basket trial. J Clin Oncol. 2019;37(15):6001.
Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17(6):738–46.
Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28(7):1124–30.
Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382(25):2419–30.
Gatzemeier U, Groth G, Butts C, Van Zandwijk N, Shepherd F, Ardizzoni A, et al. Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann Oncol. 2004;15(1):19–27.
Meric-Bernstam F, Hainsworth J, Bose R, Burris Iii HA, Friedman CF, Kurzrock R, et al. MyPathway HER2 basket study: Pertuzumab (P) + trastuzumab (H) treatment of a large, tissue-agnostic cohort of patients with HER2-positive advanced solid tumors. J Clin Oncol. 2021;39(15):3004.
Peters S, Stahel R, Bubendorf L, Bonomi P, Villegas A, Kowalski DM, et al. Trastuzumab Emtansine (T-DM1) in Patients with Previously Treated HER2-Overexpressing Metastatic Non-Small Cell Lung Cancer: Efficacy, Safety, and Biomarkers. Clin Cancer Res. 2019;25(1):64–72.
Nakagawa K, Nagasaka M, Felip E, Pacheco J, Baik C, Goto Y, et al. OA04.05 Trastuzumab Deruxtecan in HER2-Overexpressing Metastatic Non-Small Cell Lung Cancer: Interim Results of DESTINY-Lung01. J Thoracic Oncol. 2021;16(3):S109–10.
Li BT, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazieres J, et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med. 2022;386(3):241–51.
Li BT, Shen R, Buonocore D, Olah ZT, Ni A, Ginsberg MS, et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: results from a Phase II basket trial. J Clin Oncol. 2018;36(24):2532–7.
Li BT, Michelini F, Misale S, Cocco E, Baldino L, Cai Y, et al. HER2-Mediated Internalization of Cytotoxic Agents in ERBB2 Amplified or Mutant Lung Cancers. Cancer Discov. 2020;10(5):674–87.
Yagisawa M, Nakamura Y, Yoshino T, Komatsu Y, Kadowaki S, Muro K, et al. A basket trial of trastuzumab deruxtecan, a HER2-targeted antibody-drug conjugate, for HER2-amplified solid tumors identified by circulating tumor DNA analysis (HERALD trial). J Clin Oncol. 2020;38(15_suppl):TPS3650-TPS.
Li BT, Meric-Bernstam F, Puvvada SD, Rowbottom J, Jolliffe D, Gustavson M, et al. A phase 2, multicenter, open-label study evaluating trastuzumab deruxtecan (T-DXd) for the treatment of solid tumors harboring specific HER2-activating mutations (DESTINY-PanTumor01). J Clin Oncol. 2021;39(15_suppl):TPS3162-TPS.
Krop IE, Ramos J, Zhang C, Hamilton EP. HER2CLIMB-04: Phase 2 open label trial of tucatinib plus trastuzumab deruxtecan in patients with HER2+ unresectable locally advanced or metastatic breast cancer with and without brain metastases (trial in progress). J Clin Oncol. 2021;39(15_suppl):TPS1097-TPS.
Carneiro BA, Bestvina CM, Shmueli ES, Gan HK, Beck JT, Robinson R, et al. Phase I study of the antibody-drug conjugate ABBV-321 in patients with non-small cell lung cancer and squamous head and neck cancer with overexpression of the epidermal growth factor receptor. J Clin Oncol. 2020;38(15_suppl):TPS3649-TPS.
Janne PA, Baik CS, Su W-C, Johnson ML, Hayashi H, Nishio M, et al. Efficacy and safety of patritumab deruxtecan (HER3-DXd) in EGFR inhibitor-resistant, EGFR-mutated (EGFRm) non-small cell lung cancer (NSCLC). J Clin Oncol. 2021;39(15):9007.
Ferraro E, Drago JZ, Modi S. Implementing antibody-drug conjugates (ADCs) in HER2-positive breast cancer: state of the art and future directions. Breast Cancer Res. 2021;23(1):84.
Banerji U, van Herpen CML, Saura C, Thistlethwaite F, Lord S, Moreno V, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20(8):1124–35.
Acknowledgments
The authors thank all the patients who participated and their families.
Funding Sources
MOBILITY3 was an investigator-initiated trial at Princess Margaret Cancer Centre, University Health Network, Toronto, Canada. Partial funding support was provided by Boehringer-Ingelheim (BI), who also kindly provided the study drug, afatinib. BI was given the opportunity to review the manuscript for medical and scientific accuracy as it relates to BI substances, as well as intellectual property considerations. BI had no role in the design, analysis or interpretation of the results in this study.
Conflict of Interest Disclosures
AR Hansen has received research funding from Karyopharm Therapeutics, Merck, Bristol Myers Squibb, Boehringer Ingelheim, GlaxoSmithKline, Roche/Genentech, Janssen, AstraZeneca/MedImmune, Astellas Pharma, BioNTech, Pfizer/EMD Serono, and Neoleukin Therapeutics; and advisory board/consulting fees from Merck, GlaxoSmithKline, Bristol Myers Squibb, Eisai, Novartis, and AstraZeneca. A Spreafico has received research funding from Novartis, Bristol-Myers Squibb, Symphogen AstraZeneca/Medimmune, Merck, Bayer, Surface Oncology, Northern Biologics, Janssen Oncology/Johnson & Johnson, Roche, Regeneron, Alkermes, Array Biopharma/Pfizer, GSK, Treadwell, Amgen; and advisory board/consulting fees from Merck, Bristol-Myers Squibb, Novartis, Oncorus, Janssen, Medison, and Immunocore. J Doi has received honorarium for educatiuonal presentation from Merck Cancda Inc. P Bedard has received research funding from Bristol-Myers Squibb, Sanofi, AstraZeneca, Geenetech/Roche, SERVIER, GlaxoSmithKline, Novartis, PTC Therapeutics, Nektar, Merck, Seattle Genetics, Mersana, Immunomedics, Lilly, Amgen, and Bicara; and advisory board/consulting fees from Seattle Genetics, Lilly, Amgen, Merck, Gilead Sciences, Bristol-Myers Squibb, Pfizer. LL Siu has received research funding from Novartis, Bristol-Myers Squibb, Pfizer, GlaxoSmithKline, Roche/Genentech, Karyopharm, AstraZeneca/Medimmune, Merck, Celgene, Astellas, Bayer, Abbvie, Amgen, Symphogen, Intensity Therapeutics, Mirati, Shattucks, and Avid; and advisory board/consulting fees from Merck, Pfizer, AstraZeneca, Roche, Symphogen, GSK, Voronoi, Treadwell Therapeutics, Arvinas, Tessa, Navire, Relay, Rubius, Janpix, Daiichi Sankyo, Coherus, Amgen, Marengo; stock ownership at Agios; and spouse has finiancial interest in Treadwell Therapeutics. AR Abdul Razak has received research funding from Deciphera, Karyopharm Therapeutics, Pfizer, Roche/Genentech, Bristol-Myers Squibb, MedImmune, Amgen, GlaxoSmithKline, Blueprint Medicines, Merck, Abbvie, Adaptimmune, Iterion Therapeutics, 23&Me, Rain Therapeutics, Neoleukin Therapeutics, Daiichi Sankyo, Symphogen; and advisory board/consulting fees from Adaptimmune, Bayer, GlaxoSmithKline; and payment for epert testimony from Medison. Other co-authors have no disclosures.
Authors’ Contributions
Conception and Design of Study: Abdul Razak, Siu; Acquisition of Data: Hansen, Spreafico, Webster, Bedard, Doi, Siu, Abdul Razak; Analysis and/or Interpretation of Data: Salawu, Hansen, Spreafico, Al-ezzi, Bedard, Wang, Siu, Abdul Razak, Approval of the version of the manuscript to be published: Salawu, Hansen, Spreafico, Al-ezzi, Webster, Bedard, Doi, Wang, Siu, Abdul Razak.
Ethics Approval and Informed Consent
The study protocol was approved by the Research Ethics Board (REB) at the Princess Margaret Cancer Centre, Toronto. All participants gave written informed consent, and the trial was performed in compliance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Data Availability
Deidentified participant data that underlie the results presented in this article and the study protocol may be requested by contacting the corresponding author.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salawu, A., Hansen, A.R., Spreafico, A. et al. A Phase 2 Trial of Afatinib in Patients with Solid Tumors that Harbor Genomic Aberrations in the HER family: The MOBILITY3 Basket Study. Targ Oncol 17, 271–281 (2022). https://doi.org/10.1007/s11523-022-00884-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-022-00884-z