Abstract
Background
Regorafenib and trifluridine/tipiracil are standard third-line chemotherapies for colorectal cancer patients, but their efficacy is limited. Anti-epidermal growth factor receptor antibody rechallenge has been reported to be promising for patients who have obtained clinical benefit from first-line cetuximab-based chemotherapy. Moreover, panitumumab showed non-inferior efficacy to cetuximab.
Objective
This study assessed the efficacy and safety of third-line panitumumab rechallenge in patients with metastatic KRAS exon 2 wild-type metastatic colorectal cancer who obtained clinical benefit from first-line panitumumab-based chemotherapy.
Patients and Methods
This was a prospective, multicenter, phase II trial conducted from October 2013 to August 2017. Major eligibility criteria included KRAS exon 2 wild-type and achievement of complete response, partial response, or continued stable disease for at least 6 months in first-line panitumumab-based therapy. Irinotecan plus panitumumab treatment was continued until disease progression or unacceptable toxicity was observed. The primary endpoint was the 3-month progression-free survival (PFS) rate.
Results
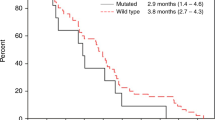
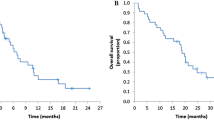
Twenty-five patients were enrolled in this study. Their median age was 66.5 years, and the 3-month PFS rate was 50.0% (95% confidence interval 30.0–70.0). The median PFS and overall survival were 3.1 months and 8.9 months, respectively. The response rate and disease control rate were 8.3% and 50.0%, respectively. Common grade 3/4 adverse events were acneiform rash (17%), hypomagnesemia (13%), and dry skin (13%). No treatment-related deaths occurred.
Conclusion
Irinotecan plus panitumumab rechallenge is a promising third-line treatment regimen in patients with metastatic wild-type KRAS colorectal cancer.
Clinical Trial Identification
UMIN000015916.




Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34.
Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17.
Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–34.
Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697–705.
Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45.
Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–8.
Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–64.
Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–422.
National Comprehensive Cancer Network. NCCN guidelines for colon cancer (version 4.2020). https://www.nccn.org/professionals/physsionals/physician_gls/pdf/colon.pdf/. Accessed 20 Jun 2020.
Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1–42.
Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–12.
Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909–19.
Yoshino T, Mizunuma N, Yamazaki K, Nishina T, Komatsu Y, Baba H, et al. TAS-102 monotherapy for pretreated metastatic colorectal cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2012;13:993–1001.
Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619–29.
Santini D, Vincenzi B, Addeo R, Garufi C, Masi G, Scartozzi M, et al. Cetuximab rechallenge in metastatic colorectal cancer patients: how to come away from acquired resistance? Ann Oncol. 2012;23:2313–8.
Cremolini C, Rossini D, Dell’Aquila E, Lonardi S, Conca E, Del Re M, et al. Rechallenge for patients with RAS and BRAF wild-type metastatic colorectal cancer with acquired resistance to first-line cetuximab and irinotecan: a phase 2 single-arm clinical trial. JAMA Oncol. 2019;5:343–50.
Osawa H, Shinozaki E, Nakamura M, Ohhara Y, Shindo Y, Shiozawa M, et al. Phase II study of cetuximab rechallenge in patients with ras wild-type metastatic colorectal cancer: E-rechallenge trial. Ann Oncol. 2018;29(8_suppl):481P.
Price TJ, Peeters M, Kim TW, Li J, Cascinu S, Ruff P, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol. 2014;15:569–79.
Hata A, Katakami N, Kitajima N. Successful cetuximab therapy after failure of panitumumab rechallenge in a patient with metastatic colorectal cancer: restoration of drug sensitivity after anti-EGFR monoclonal antibody-free interval. J Gastrointest Cancer. 2014;45:506–7.
Masuishi T, Tsuji A, Kotaka M, Nakamura M, Kochi M, Takagane A, et al. Phase 2 study of irinotecan plus cetuximab rechallenge as third-line treatment in KRAS wild-type metastatic colorectal cancer: JACCRO CC-08. Br J Cancer. 2020;123:1490–5.
Chong LC, Hardingham JE, Townsend AR, Piantadosi C, Rico GT, Karapetis C, et al. Rechallenge with anti-EGFR therapy in metastatic colorectal cancer (mCRC): results from South Australia mCRC registry. Target Oncol. 2020;15:751–7.
Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486:532–6.
Diaz LA Jr, Williams RT, Wu J, Kinde I, Hecht JR, Berlin J, et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486:537–40.
Siravegna G, Mussolin B, Buscarino M, Corti G, Cassingena A, Crisafulli G, et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med. 2015;21:795–801.
Parseghian CM, Napolitano S, Loree JM, Kopetz S. Mechanisms of innate and acquired resistance to anti-EGFR therapy: a review of current knowledge with a focus on rechallenge therapies. Clin Cancer Res. 2019;25:6899–908.
Sunakawa Y, Nakamura M, Ishizaki M, Kataoka M, Satake H, Kitazono M, et al. RAS mutations in circulating tumor DNA and clinical outcomes of rechallenge treatment with anti-EGFR antibodies in patients with metastatic colorectal cancer. JCO Precis Oncol. 2020;4:898–911.
Satake H, Sunakawa Y, Miyamoto Y, Nakamura M, Nakayama H, Shiozawa M, et al. A phase II trial of 1st-line modified-FOLFOXIRI plus bevacizumab treatment for metastatic colorectal cancer harboring RAS mutation: JACCRO CC-11. Oncotarget. 2018;9:18811–20.
Acknowledgements
The authors would like to thank the patients and their families, their collaborators who contributed to the study and recruited patients, and the members of the Japan Clinical Cancer Research Organization (JACCRO) Data Center and Support Office. The authors would also like to thank Yasuhiro Shimada, Kenji Omura, and Toru Takebayashi for the independent data and safety monitoring committee.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the JACCRO and funded by Takeda Pharmaceutical Co., Ltd.
Conflicts of interest
A.T. and W.I. have received grants from Takeda Pharmaceutical Co., Ltd. M.N., T.W., D.M., H.M., M.K., M.T., and M.F. declare that they have no conflicts of interest that might be relevant to the contents of this article.
Ethics approval
This study has been reviewed and approved by the JACCRO Institutional Review Board (No.2013-7).
Consent to participate
Written, informed consent was obtained from all study participants.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
AT, WI, and MF analyzed and interpreted patient data and contributed significantly to the writing of the manuscript. All authors enrolled patients, treated them, participated in manuscript preparation, and read and approved the final manuscript.
Rights and permissions
About this article
Cite this article
Tsuji, A., Nakamura, M., Watanabe, T. et al. Phase II Study of Third-Line Panitumumab Rechallenge in Patients with Metastatic Wild-Type KRAS Colorectal Cancer Who Obtained Clinical Benefit from First-Line Panitumumab-Based Chemotherapy: JACCRO CC-09. Targ Oncol 16, 753–760 (2021). https://doi.org/10.1007/s11523-021-00845-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-021-00845-y