Abstract
Background
Bone metastases (BM) in renal cell carcinoma (RCC) patients are associated with poor outcomes. There are limited published data on outcomes in these patients with immunotherapy agents. We present a multi-institutional, retrospective analysis of metastatic RCC patients with BM treated with ipilimumab and nivolumab (I + N).
Objective
Patient, tumor, and treatment-related variables were retrospectively collected from electronic medical records of patients with a histologically confirmed diagnosis of RCC and at least one radiographically confirmed BM prior to initiation of I + N. Best objective response was assessed by clinical chart review, imaging reports, and treating physician evaluation; progression-free survival (PFS) and overall survival (OS) were recorded as of 31 December 2020. Descriptive statistics were used to summarize patient characteristics and BM-related variables. Kaplan-Meier method and Mantel-Haenszel log-rank test were used to compare survival among groups. Cox regression univariable and multivariable models were used to correlate patient- and treatment-related variables to outcomes.
Results
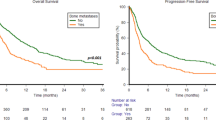
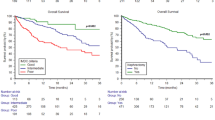
Eighty patients with RCC and BM treated with I + N were identified. Patients were predominantly male and Caucasian presenting primarily with IMDC intermediate or poor-risk clear-cell RCC. Best response to I + N was progressive disease (46%), stable disease (28%), partial response (21%), and not evaluable (5%). Median PFS was 6.1 months (95% CI 3.8–8.9 months) with the majority of patients (65%) discontinuing I + N due to disease progression. Median OS was 25.6 months (95% CI 14.9–NA) with median follow-up of 25.2 months. A multivariable regression model for PFS showed several variables to be significantly associated with worse PFS including female gender [p = 0.02; hazard ratio (HR) 2.16; 95% CI 1.14–4.12], metastases to other sites (p = 0.006; HR 2.12; 95% CI 1.24–3.62) and presence of BM to ribs (p = 0.0007; HR 2.61; 95% CI 1.50–4.52). A multivariable Cox model of OS showed no prior radiation therapy to BM (p = 0.02; HR 2.17; 95% CI 1.13–4.17) and presence of liver metastases (p = 0.0006; HR 3.19; 95% CI 1.65–6.19) to be significantly associated with worse OS.
Conclusion
RCC patients with ≥ 1 BM who received I + N therapy had a relatively low response rate, PFS, and OS. Strategies to improve outcomes in this subset of patients are needed.
Access this article
We’re sorry, something doesn't seem to be working properly.
Please try refreshing the page. If that doesn't work, please contact support so we can address the problem.


Similar content being viewed by others
References
Institute NC. SEER Cancer Stat Facts: Kidney and Renal Pelvis Cancer. National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/statfacts/html/kidrp.html
Wood SL, Brown JE. Skeletal metastasis in renal cell carcinoma: current and future management options. Cancer Treat Rev. 2012;38(4):284–91.
Woodward E, Jagdev S, McParland L, Clark K, Gregory W, Newsham A, et al. Skeletal complications and survival in renal cancer patients with bone metastases. Bone. 2011;48(1):160–6.
Ibrahim A, Scher N, Williams G, Sridhara R, Li N, Chen G, et al. Approval summary for zoledronic acid for treatment of multiple myeloma and cancer bone metastases. Clin Cancer Res. 2003;9(7):2394–9.
Zekri J, Ahmed N, Coleman RE, Hancock BW. The skeletal metastatic complications of renal cell carcinoma. Int J Oncol. 2001;19(2):379–82.
Santoni M, Conti A, Procopio G, Porta C, Ibrahim T, Barni S, et al. Bone metastases in patients with metastatic renal cell carcinoma: are they always associated with poor prognosis? J Exp Clin Cancer Res. 2015;34:10.
Beuselinck B, Oudard S, Rixe O, Wolter P, Blesius A, Ayllon J, et al. Negative impact of bone metastasis on outcome in clear-cell renal cell carcinoma treated with sunitinib. Ann Oncol. 2011;22(4):794–800.
Kalra S, Verma J, Atkinson BJ, Matin SF, Wood CG, Karam JA, et al. Outcomes of patients with metastatic renal cell carcinoma and bone metastases in the targeted therapy era. Clin Genitourin Cancer. 2017;15(3):363–70.
Kume H, Kakutani S, Yamada Y, Shinohara M, Tominaga T, Suzuki M, et al. Prognostic factors for renal cell carcinoma with bone metastasis: who are the long-term survivors? J Urol. 2011;185(5):1611–4.
McKay RR, Kroeger N, Xie W, Lee JL, Knox JJ, Bjarnason GA, et al. Impact of bone and liver metastases on patients with renal cell carcinoma treated with targeted therapy. Eur Urol. 2014;65(3):577–84.
Massari F, Di Nunno V, Guida A, Costa Silva CA, Derosa L, Mollica V, et al. Addition of primary metastatic site on bone, brain, and liver to IMDC criteria in patients with metastatic renal cell carcinoma: a validation study. Clin Genitourin Cancer. 2021;19(1):32–40.
Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1814–23.
Powles T, Motzer RJ, Escudier B, Pal S, Kollmannsberger C, Pikiel J, et al. Outcomes based on prior therapy in the phase 3 METEOR trial of cabozantinib versus everolimus in advanced renal cell carcinoma. Br J Cancer. 2018;119(6):663–9.
Choueiri TK, Escudier B, Powles T, Tannir NM, Mainwaring PN, Rini BI, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17(7):917–27.
Escudier B, Powles T, Motzer RJ, Olencki T, Aren Frontera O, Oudard S, et al. Cabozantinib, a new standard of care for patients with advanced renal cell carcinoma and bone metastases? Subgroup analysis of the METEOR trial. J Clin Oncol. 2018;36(8):765–72.
Broom RJ, Hinder V, Sharples K, Proctor J, Duffey S, Pollard S, et al. Everolimus and zoledronic acid in patients with renal cell carcinoma with bone metastases: a randomized first-line phase II trial. Clin Genitourin Cancer. 2015;13(1):50–8.
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–13.
Escudier B, Sharma P, McDermott DF, George S, Hammers HJ, Srinivas S, et al. CheckMate 025 randomized phase 3 study: outcomes by key baseline factors and prior therapy for nivolumab versus everolimus in advanced renal cell carcinoma. Eur Urol. 2017;72(6):962–71.
Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–90.
McDonald R, Ding K, Brundage M, Meyer RM, Nabid A, Chabot P, et al. Effect of radiotherapy on painful bone metastases: a secondary analysis of the NCIC clinical trials group symptom control trial SC.23. JAMA Oncol. 2017;3(7):953–9.
Grünwald V, Eberhardt B, Bex A, Flörcken A, Gauler T, Derlin T, et al. An interdisciplinary consensus on the management of bone metastases from renal cell carcinoma. Nat Rev Urol. 2018;15(8):511–21.
Wersäll PJ, Blomgren H, Lax I, Kälkner KM, Linder C, Lundell G, et al. Extracranial stereotactic radiotherapy for primary and metastatic renal cell carcinoma. Radiother Oncol. 2005;77(1):88–95.
Taunk NK, Spratt DE, Bilsky M, Yamada Y. Spine radiosurgery in the management of renal cell carcinoma metastases. J Natl Compr Cancer Netw. 2015;13(6):801–9.
Tree AC, Khoo VS, Eeles RA, Ahmed M, Dearnaley DP, Hawkins MA, et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol. 2013;14(1):e28–37.
Amini A, Altoos B, Bourlon MT, Bedrick E, Bhatia S, Kessler ER, et al. Local control rates of metastatic renal cell carcinoma (RCC) to the bone using stereotactic body radiation therapy: is RCC truly radioresistant? Pract Radiat Oncol. 2015;5(6):e589–96.
Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma : final results and analysis of prognostic factors. Cancer. 2010;116(18):4256–65.
Tumeh PC, Hellmann MD, Hamid O, Tsai KK, Loo KL, Gubens MA, et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res. 2017;5(5):417–24.
Wyld L, Gutteridge E, Pinder SE, James JJ, Chan SY, Cheung KL, et al. Prognostic factors for patients with hepatic metastases from breast cancer. Br J Cancer. 2003;89(2):284–90.
Staehler MD, Kruse J, Haseke N, Stadler T, Roosen A, Karl A, et al. Liver resection for metastatic disease prolongs survival in renal cell carcinoma: 12-year results from a retrospective comparative analysis. World J Urol. 2010;28(4):543–7.
Ma VT, Su CT, Hu M, Taylor JMG, Daignault-Newton S, Kellezi O, et al. Characterization of outcomes in patients with advanced genitourinary malignancies treated with immune checkpoint inhibitors. Urol Oncol. 2021;39(7):437.e1-437.e9.
Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–92.
Fidler IJ. The pathogenesis of cancer metastasis: the “seed and soil” hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–8.
Keizer RJ, Huitema AD, Schellens JH, Beijnen JH. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49(8):493–507.
Ovacik M, Lin K. Tutorial on monoclonal antibody pharmacokinetics and its considerations in early development. Clin Transl Sci. 2018;11(6):540–52.
Limmer A, Ohl J, Kurts C, Ljunggren HG, Reiss Y, Groettrup M, et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med. 2000;6(12):1348–54.
Crispe IN, Dao T, Klugewitz K, Mehal WZ, Metz DP. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol Rev. 2000;174:47–62.
Li F, Tian Z. The liver works as a school to educate regulatory immune cells. Cell Mol Immunol. 2013;10(4):292–302.
Rosen LS, Gordon D, Tchekmedyian S, Yanagihara R, Hirsh V, Krzakowski M, et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial—the Zoledronic acid lung cancer and other solid tumors study group. J Clin Oncol. 2003;21(16):3150–7.
Tunn UW, Stenzl A, Schultze-Seemann W, Strauss A, Kindler M, Miller K, et al. Positive effects of zoledronate on skeletal-related events in patients with renal cell cancer and bone metastases. Can J Urol. 2012;19(3):6261–7.
McKay RR, Lin X, Perkins JJ, Heng DY, Simantov R, Choueiri TK. Prognostic significance of bone metastases and bisphosphonate therapy in patients with renal cell carcinoma. Eur Urol. 2014;66(3):502–9.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Clamp A, Danson S, Nguyen H, Cole D, Clemons M. Assessment of therapeutic response in patients with metastatic bone disease. Lancet Oncol. 2004;5(10):607–16.
Sahi C, Knox JJ, Clemons M, Joshua AM, Broom R. Renal cell carcinoma bone metastases: clinical advances. Ther Adv Med Oncol. 2010;2(2):75–83.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this article.
Conflict of Interest
Dr. Kunal Desai declares the following relationship: Consulting with Tyra BioSciences and CinRx. Dr. Landon Brown declares the following relationships: Consulting with Seattle Genetics. Wei Wei declares that he has no conflicts of interest that might be relevant to the contents of this article. Dr. Matthew Tucker declares that he has no conflicts of interest that might be relevant to the contents of this article. Dr. Chester Kao declares that he has no conflicts of interest that might be relevant to the contents of this article. Dr. Emily Kinsey declares that she has no conflicts of interest that might be relevant to the contents of this article. Dr Brian Rini declares the following relationships: Research funding (to Vanderbilt University) from Pfizer, Hoffman-LaRoche, Incyte, AstraZeneca, Taris, Seattle Genetics, Arrowhead Pharmaceuticals, Immunomedics, BMS, Mirati Therapeutics, Merck, Surface Oncology, Dragonfly Therapeutics, Aravive and Exelixis; Consulting with Bristol Myer Squibb, Pfizer, Roche/Genentech, Aveo, Synthorx, Compugen, Merck, Corvus, Surface Oncology, 3DMedicines, Aravive, Alkermes, Arrowhead, GSK, Shionogi, and Eisai. Stock ownership in PTC Therapeutics. Dr. Kathryn Beckermann declares the following relationships: Research funding (to Vanderbilt) from BMS-IASLC-LCFA for a young investigator award. Consulting with Exelexis. Dr. Tian Zhang declares the following relationships: Research funding (to Duke University) from Pfizer, Janssen, Acerta, Abbvie, Novartis, Merrimack, OmniSeq, PGDx, Merck, Mirati, Astellas, and Genentech; Speaking with Genomic Health and Sanofi Aventis; Consulting with AstraZeneca, Bayer, Pfizer, Foundation Medicine, Janssen, Merck, Amgen, MJH Associates, and BMS. Stock ownership/employment (spouse) from Capio Biosciences, Archimmune Therapeutics and Nanorobotics. Dr. Moshe Ornstein declares the following relationships: Consulting with BMS, Merck, Pfizer, Aveo, Exelixis, and Eisai.
Ethics approval
Collection and analysis of patient-level data for this article was approved by the participating institutions (Cleveland Clinic IRB-approved protocol 19-609, Duke University IRB-approved protocol Pro00101984, and Vanderbilt University IRB-approved protocol 160979).
Consent to participate
The need for written consent from patients was waived per above approval protocols.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Authors’ Contribution
Dr. Desai wrote the majority of the content of the manuscript and collected patient data for Cleveland Clinic. Drs. Brown and Tucker edited the manuscript and collected patient data at Duke University and Vanderbilt University, respectively. Mr. Wei Wei conducted the statistical analysis for this article and wrote the statistics-related content. Drs. Kao and Kinsey collected patient data for Duke University. Drs. Rini, Beckermann, Zhang, and Ornstein provided editorial input for the article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Desai, K., Brown, L., Wei, W. et al. A Multi-institutional, Retrospective Analysis of Patients with Metastatic Renal Cell Carcinoma to Bone Treated with Combination Ipilimumab and Nivolumab. Targ Oncol 16, 633–642 (2021). https://doi.org/10.1007/s11523-021-00832-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-021-00832-3