Abstract
Background
Leptomeningeal metastasis (LM) is a fatal complication of advanced non-small-cell lung cancer (NSCLC).
Objective
The aim of this study was to evaluate the utility of cerebrospinal fluid (CSF) as a medium for epidermal growth factor receptor (EGFR) mutation testing in clinical practice.
Patients and methods
We prospectively enrolled patients with EGFR-mutant NSCLC who underwent CSF sampling for suspected LM. The supernatant of CSF after routine cytology examination was collected. The diagnosis of LM was established according to EANO-ESMO criteria. CSF and plasma cell-free DNA (cfDNA) were retrieved for EGFR mutation testing.
Results
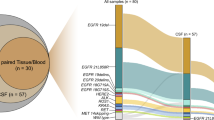
Fifty-one patients with a median age of 62.7 years were enrolled. The median duration from initial diagnosis to CSF sampling was 23.0 months and most patients (94.1%) had received at least one EGFR-tyrosine kinase inhibitor. Adenocarcinoma cells were found in 37 CSF samples (72.5%), and 48 (94.1%) patients had confirmed or probable LM. Thirty-five of these 48 patients (72.9%) had valid EGFR mutation-testing results using CSF cfDNA and tended to have higher white blood cell counts and positive cytology in their CSF compared to those with invalid mutation testing results. The overall detection rate of EGFR mutation in CSF cfDNA was 68.8%, and the T790M detection rate was 14.6%. In 37 patients with paired CSF and plasma samples, the concordance rate of the EGFR mutation results was 29.7%.
Conclusions
For patients with EGFR-mutant NSCLC with LM, CSF supernatant is a valuable source for EGFR mutation testing and may provide important information.


Similar content being viewed by others
References
Remon J, Le Rhun E, Besse B. Leptomeningeal carcinomatosis in non-small cell lung cancer patients: a continuing challenge in the personalized treatment era. Cancer Treat Rev. 2017;53:128–37. https://doi.org/10.1016/j.ctrv.2016.12.006.
Li YS, Jiang BY, Yang JJ, Tu HY, Zhou Q, Guo WB, et al. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol. 2016;11(11):1962–9. https://doi.org/10.1016/j.jtho.2016.06.029.
Liao BC, Lee JH, Lin CC, Chen YF, Chang CH, Ho CC, et al. Epidermal growth factor receptor tyrosine kinase inhibitors for non-small-cell lung cancer patients with leptomeningeal carcinomatosis. J Thorac Oncol. 2015;10(12):1754–61. https://doi.org/10.1097/JTO.0000000000000669.
Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol. 2018;19(1):e43–55. https://doi.org/10.1016/s1470-2045(17)30689-7.
Chamberlain MC. Leptomeningeal metastasis. Curr Opin Oncol. 2010;22(6):627–35. https://doi.org/10.1097/CCO.0b013e32833de986.
Mandel P, Metais P. Nuclear acids in human blood plasma. C R Seances Soc Biol Fil. 1948;142(3–4):241–3.
Sidransky D. Nucleic acid-based methods for the detection of cancer. Science. 1997;278(5340):1054–9.
Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra24. https://doi.org/10.1126/scitranslmed.3007094.
Mok T, Wu YL, Lee JS, Yu CJ, Sriuranpong V, Sandoval-Tan J, et al. Detection and dynamic changes of EGFR mutations from circulating tumor DNA as a predictor of survival outcomes in NSCLC patients treated with first-line intercalated erlotinib and chemotherapy. Clin Cancer Res. 2015;21(14):3196–203. https://doi.org/10.1158/1078-0432.ccr-14-2594.
Yung TK, Chan KC, Mok TS, Tong J, To KF, Lo YM. Single-molecule detection of epidermal growth factor receptor mutations in plasma by microfluidics digital PCR in non-small cell lung cancer patients. Clin Cancer Res. 2009;15(6):2076–84. https://doi.org/10.1158/1078-0432.ccr-08-2622.
De Mattos-Arruda L, Mayor R, Ng CK, Weigelt B, Martinez-Ricarte F, Torrejon D, et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun. 2015;6:8839. https://doi.org/10.1038/ncomms9839.
Pentsova EI, Shah RH, Tang J, Boire A, You D, Briggs S, et al. Evaluating cancer of the central nervous system through next-generation sequencing of cerebrospinal fluid. J Clin Oncol. 2016;34(20):2404–15. https://doi.org/10.1200/jco.2016.66.6487.
Le Rhun E, Weller M, Brandsma D, Van den Bent M, de Azambuja E, Henriksson R, et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol. 2017;28(suppl_4):vi84–99. https://doi.org/10.1093/annonc/mdx221.
Nayar G, Ejikeme T, Chongsathidkiet P, Elsamadicy AA, Blackwell KL, Clarke JM, et al. Leptomeningeal disease: current diagnostic and therapeutic strategies. Oncotarget. 2017;8(42):73312–28. https://doi.org/10.18632/oncotarget.20272.
Li YS, Jiang BY, Yang JJ, Zhang XC, Zhang Z, Ye JY, et al. Unique genetic profiles from cerebrospinal fluid cell-free DNA in leptomeningeal metastases of EGFR-mutant non-small-cell lung cancer: a new medium of liquid biopsy. Ann Oncol. 2018;29(4):945–52. https://doi.org/10.1093/annonc/mdy009.
Huang R, Xu X, Li D, Chen K, Zhan Q, Ge M, et al. Digital PCR-based detection of EGFR mutations in paired plasma and CSF samples of lung adenocarcinoma patients with central nervous system metastases. Target Oncol. 2019;14(3):343–50. https://doi.org/10.1007/s11523-019-00645-5.
Suryavanshi M, Jaipuria J, Panigrahi MK, Goyal N, Singal R, Mehta A, et al. CSF cell-free DNA EGFR testing using DdPCR holds promise over conventional modalities for diagnosing leptomeningeal involvement in patients with non-small cell lung cancer. Lung Cancer. 2020;148:33–9. https://doi.org/10.1016/j.lungcan.2020.07.034.
Kawahara A, Abe H, Murata K, Ishii H, Azuma K, Takase Y, et al. Screening system for epidermal growth factor receptor mutation detection in cytology cell-free DNA of cerebrospinal fluid based on assured sample quality. Cytopathology. 2019;30(2):144–9. https://doi.org/10.1111/cyt.12660.
Moss J, Magenheim J, Neiman D, Zemmour H, Loyfer N, Korach A, et al. Comprehensive human cell-type methylation atlas reveals origins of circulating cell-free DNA in health and disease. Nat Commun. 2018;9(1):5068. https://doi.org/10.1038/s41467-018-07466-6.
Socinski MA, Villaruz LC, Ross J. Understanding mechanisms of resistance in the epithelial growth factor receptor in non-small cell lung cancer and the role of biopsy at progression. Oncologist. 2017;22(1):3–11. https://doi.org/10.1634/theoncologist.2016-0285.
Nanjo S, Arai S, Wang W, Takeuchi S, Yamada T, Hata A, et al. MET copy number gain is associated with gefitinib resistance in leptomeningeal carcinomatosis of EGFR-mutant lung cancer. Mol Cancer Ther. 2017;16(3):506–15. https://doi.org/10.1158/1535-7163.MCT-16-0522.
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–40. https://doi.org/10.1056/NEJMoa1612674.
Aldea M, Hendriks L, Mezquita L, Jovelet C, Planchard D, Auclin E, et al. Circulating tumor DNA analysis for patients with oncogene-addicted NSCLC with isolated central nervous system progression. J Thorac Oncol. 2020;15(3):383–91. https://doi.org/10.1016/j.jtho.2019.11.024.
Nanjo S, Hata A, Okuda C, Kaji R, Okada H, Tamura D, et al. Standard-dose osimertinib for refractory leptomeningeal metastases in T790M-positive EGFR-mutant non-small cell lung cancer. Br J Cancer. 2018;118(1):32–7. https://doi.org/10.1038/bjc.2017.394.
Ahn MJ, Chiu CH, Cheng Y, Han JY, Goldberg SB, Greystoke A, et al. Osimertinib for patients with leptomeningeal metastases associated with EGFR T790M-positive advanced NSCLC: the AURA leptomeningeal metastases analysis. J Thorac Oncol. 2020;15(4):637–48. https://doi.org/10.1016/j.jtho.2019.12.113.
Yang JCH, Kim SW, Kim DW, Lee JS, Cho BC, Ahn JS, et al. Osimertinib in patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer and leptomeningeal metastases: the BLOOM study. J Clin Oncol. 2020;38(6):538–47. https://doi.org/10.1200/jco.19.00457.
Goldman JW, Noor ZS, Remon J, Besse B, Rosenfeld N. Are liquid biopsies a surrogate for tissue EGFR testing? Ann Oncol. 2018;29(suppl_1):i38–46. https://doi.org/10.1093/annonc/mdx706.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the Ministry of Science and Technology, Taiwan (Grant number MOST106-2314-B-075-031-MY), Ministry of Health and Welfare, Taiwan (Grant number MOHW109-TDU-B-211-134019), and Taipei Veterans General Hospital (Grant number V110B-008).
Conflict of interest
CLC has received honoraria from AstraZeneca, Boehringer Ingelheim, and Roche. YHL has received honoraria from AstraZeneca, Boehringer Ingelheim, and Pfizer. THS has received honoraria from Boehringer Ingelheim and Novartis. YMC has received honoraria from Boehringer Ingelheim, Eli Lilly, Roche/Genentech/Chugai, MSD, Pfizer, Novartis, BMS, Ono Pharmaceutical, AstraZeneca, and Takeda Oncology; and served as advisor for Boehringer Ingelheim, Eli Lilly, Roche/Chugai, MSD, AstraZeneca, and Takeda Oncology. CHC has received honoraria from AstraZeneca, Boehringer Ingelheim, Pfizer, and Roche. The other authors declare no conflicts of interest that might be relevant to the contents of this article.
Ethics approval
The Institutional Review Board of Taipei Veterans General Hospital approved this study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Data availability
Not applicable.
Code availability
Not applicable.
Author contributions
Conceptualization: CLC, CHC, YMC, TYC. Data collection, experiment, and analysis: CCL, CHW, HCH, YCY, CIS, YHL, THS. Statistics: CLC, HJC, YTH. First draft preparation: CLC, CHC. Review and final approval: all authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chiang, CL., Lee, CC., Huang, HC. et al. Utility of Cerebrospinal Fluid Cell-Free DNA in Patients with EGFR-Mutant Non-Small-Cell Lung Cancer with Leptomeningeal Metastasis. Targ Oncol 16, 207–214 (2021). https://doi.org/10.1007/s11523-021-00791-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-021-00791-9