Abstract
Background
Crizotinib has been approved for the treatment of non-small-cell lung cancer (NSCLC) with ROS proto-oncogene 1 (ROS1) gene fusion. This drug has also been granted breakthrough designation for NSCLCs with MET exon 14 alterations.
Objective
This systematic review and meta-analysis aimed to investigate the efficacy and safety of crizotinib in patients with these diseases.
Methods
We searched PubMed and Web of Science for relevant studies. Meta-analysis of proportions was conducted to calculate the pooled rate of complete response, partial response, stable disease, progressive disease, disease control rate (DCR), objective response rate (ORR), and drug adverse effects (AEs) of crizotinib in NSCLCs with ROS1 rearrangement or MET alterations.
Results
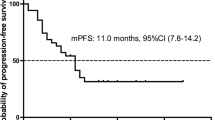
A total of 20 studies were included for meta-analysis. Among patients with ROS1-positive NSCLC, crizotinib exhibited a pooled DCR of 93.2% (95% confidence interval [CI] 90.8–95.5) and a pooled ORR of 77.4% (95% CI 72.8–82.1). The median progression-free survival (PFS) and overall survival (OS) of patients in this group was 14.5 and 32.6 months, respectively. For NSCLC with MET alterations, crizotinib was associated with a lower efficacy (DCR 78.9% [95% CI 70.3–87.4] and ORR 40.6% [95% CI 28.3–53.0]). The median PFS was 5.2 months, and median OS was 12.7 months. The most common drug AEs were vision impairment (43.7%), edema (42.9%), and fatigue (40.1%).
Conclusion
Our study highlighted and confirmed the efficacy of crizotinib in patients with NSCLC with ROS1 or MET genetic alterations. Crizotinib had remarkable effects on advanced NSCLC with ROS1 fusion, as previously reported. However, the role of this targeted therapy in MET-altered NSCLC remains investigational.



Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–39. https://doi.org/10.1056/nejmoa040938.
Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352(8):786–92. https://doi.org/10.1056/nejmoa044238.
Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–6. https://doi.org/10.1038/nature05945.
Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–94. https://doi.org/10.1056/nejmoa1214886.
Bergethon K, Shaw AT, Ou SH, Katayama R, Lovly CM, McDonald NT, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30(8):863–70. https://doi.org/10.1200/jco.2011.35.6345.
Shaw AT, Ou SHI, Bang YJ, Camidge DR, Solomon BJ, Salgia R, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371(21):1963–71. https://doi.org/10.1056/nejmoa1406766.
Caparica R, Yen CT, Coudry R, Ignatius SH, Varella-Garcia M, Camidge DR, et al. Responses to crizotinib can occur in high-level MET-amplified non-small cell lung cancer independent of MET exon 14 alterations. J Thorac Oncol. 2017;12(1):141–4. https://doi.org/10.1016/j.jtho.2016.09.116.
Landi L, Chiari R, Tiseo M, D’Inca F, Dazzi C, Chella A, et al. Crizotinib in MET-deregulated or ROS1-rearranged pretreated non-small cell lung cancer (METROS): a phase II, prospective, multicenter, two-arms trial. Clin Cancer Res. 2019;25(24):7312–9. https://doi.org/10.1158/1078-0432.ccr-19-0994.
Ou SH, Tan J, Yen Y, Soo RA. ROS1 as a ‘druggable’ receptor tyrosine kinase: lessons learned from inhibiting the ALK pathway. Expert Rev Anticancer Ther. 2012;12(4):447–56. https://doi.org/10.1586/era.12.17.
Sadiq AA, Salgia R. MET as a possible target for non-small-cell lung cancer. J Clin Oncol. 2013;31(8):1089–96. https://doi.org/10.1200/jco.2012.43.9422.
Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science (NY NY). 2007;316(5827):1039–43. https://doi.org/10.1126/science.1141478.
Bean J, Brennan C, Shih J-Y, Riely G, Viale A, Wang L, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci. 2007;104(52):20932. https://doi.org/10.1073/pnas.0710370104.
Onozato R, Kosaka T, Kuwano H, Sekido Y, Yatabe Y, Mitsudomi T. Activation of MET by gene amplification or by splice mutations deleting the juxtamembrane domain in primary resected lung cancers. J Thorac Oncol. 2009;4(1):5–11. https://doi.org/10.1097/jto.0b013e3181913e0e.
Lin JJ, Shaw AT. Recent advances in targeting ROS1 in lung cancer. J Thorac Oncol. 2017;12(11):1611–25. https://doi.org/10.1016/j.jtho.2017.08.002.
Awad MM, Oxnard GR, Jackman DM, Savukoski DO, Hall D, Shivdasani P, et al. MET exon 14 mutations in non-small-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-Met overexpression. J Clin Oncol. 2016;34(7):721. https://doi.org/10.1200/jco.2015.63.4600.
Tong JH, Yeung SF, Chan AWH, Chung LY, Chau SL, Lung RWM, et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin Cancer Res. 2016;22(12):3048–56. https://doi.org/10.1158/1078-0432.ccr-15-2061.
Vuong HG, Ho ATN, Altibi AMA, Nakazawa T, Katoh R, Kondo T. Clinicopathological implications of MET exon 14 mutations in non-small cell lung cancer—a systematic review and meta-analysis. Lung Cancer. 2018;123:76–82. https://doi.org/10.1016/j.lungcan.2018.07.006.
Kong-Beltran M, Seshagiri S, Zha J, Zhu W, Bhawe K, Mendoza N, et al. Somatic mutations lead to an oncogenic deletion of met in lung cancer. Cancer Res. 2006;66(1):283–9. https://doi.org/10.1158/0008-5472.can-05-2749.
Kazandjian D, Blumenthal GM, Chen H-Y, He K, Patel M, Justice R, et al. FDA approval summary: crizotinib for the treatment of metastatic non-small cell lung cancer with anaplastic lymphoma kinase rearrangements. Oncologist. 2014;19(10):e5.
FDA expands use of Xalkori to treat rare form of advanced non-small cell lung cancer. US Food & Drug Administration. 2016. https://www.fda.gov/news-events/press-announcements/fda-expands-use-xalkori-treat-rare-form-advanced-non-small-cell-lung-cancer. Accessed 15 July 2020.
PFIZER’S XALKORI® (CRIZOTINIB) RECEIVES FDA BREAKTHROUGH THERAPY DESIGNATION IN TWO NEW INDICATIONS. Pfizer. 2018. https://www.pfizer.com/news/press-release/press-release-detail/pfizer_s_xalkori_crizotinib_receives_fda_breakthrough_therapy_designation_in_two_new_indications-0. Accessed 15 July 2020.
Wiesweg M, Schuler M, Schildhaus HU. Crizotinib in ROS1 and MET deregulated NSCLC-letter. Clin Cancer Res. 2020;26(7):1774. https://doi.org/10.1158/1078-0432.ccr-19-3740.
Shimokawa M, Nosaki K, Seto T, Ohashi K, Morise M, Horinouchi H, et al. Phase II, open-label, multicenter trial of crizotinib in Japanese patients with advanced non-small cell lung cancer harboring a MET gene alteration: Co-MET study. Trials. 2020;21(1):298. https://doi.org/10.1186/s13063-020-4221-7.
Drilon A, Clark JW, Weiss J, Ou SHI, Camidge DR, Solomon BJ, et al. Antitumor activity of crizotinib in lung cancers harboring a MET exon 14 alteration. Nat Med. 2020;26(1):47. https://doi.org/10.1038/s41591-019-0716-8.
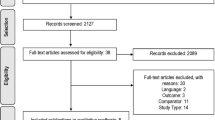
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Capizzi E, Dall’Olio FG, Gruppioni E, Sperandi F, Altimari A, Giunchi F, et al. Clinical significance of ROS1 5′ deletions in non-small cell lung cancer. Lung Cancer. 2019;135:88–91. https://doi.org/10.1016/j.lungcan.2019.07.017.
Joshi A, Pande N, Noronha V, Patil V, Kumar R, Chougule A, et al. ROS1 mutation non-small cell lung cancer-access to optimal treatment and outcomes. Ecancermedicalscience. 2019;13:900. https://doi.org/10.3332/ecancer.2019.900.
Li Z, Shen L, Ding D, Huang J, Zhang J, Chen Z, et al. Efficacy of crizotinib among different types of ROS1 fusion partners in patients with ROS1-rearranged non-small cell lung cancer. J Thorac Oncol. 2018;13(7):987–95. https://doi.org/10.1016/j.jtho.2018.04.016.
Liu C, Yu H, Chang J, Chen H, Li Y, Zhao W, et al. Crizotinib in Chinese patients with ROS1-rearranged advanced non-small-cell lung cancer in routine clinical practice. Target Oncol. 2019;14(3):315–23. https://doi.org/10.1007/s11523-019-00636-6.
Masuda K, Fujiwara Y, Shinno Y, Mizuno T, Sato J, Morita R, et al. Efficacy and safety of crizotinib in patients with ROS1 rearranged non-small cell lung cancer: a retrospective analysis. J Thorac Dis. 2019;11(7):2965–72. https://doi.org/10.21037/jtd.2019.07.44.
Mazières J, Zalcman G, Crinò L, Biondani P, Barlesi F, Filleron T, et al. Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. J Clin Oncol. 2015;33(9):992–9. https://doi.org/10.1200/jco.2014.58.3302.
Mehta A, Saifi M, Batra U, Suryavanshi M, Gupta K. Incidence of ROS1-rearranged non-small-cell lung carcinoma in india and efficacy of crizotinib in lung adenocarcinoma patients. Lung Cancer-Targets Therapy. 2020;11:19–25. https://doi.org/10.2147/lctt.s244366.
Michels S, Massutí B, Schildhaus HU, Franklin J, Sebastian M, Felip E, et al. Safety and efficacy of crizotinib in patients with advanced or metastatic ROS1-rearranged lung cancer (EUCROSS): a European phase II clinical trial. J Thorac Oncol. 2019;14(7):1266–76. https://doi.org/10.1016/j.jtho.2019.03.020.
Moro-Sibilot D, Cozic N, Pero M, Mazieres J, Otto J, Souquet PJ, et al. Crizotinib in c-MET- or ROS1-positive NSCLC: results of the AcSe phase II trial. Ann Oncol. 2019;30(12):1985–91. https://doi.org/10.1093/annonc/mdz407.
Song ZB, Wang H, Yu ZY, Lu PH, Xu CW, Chen G, et al. De novo MET amplification in Chinese patients with non-small-cell lung cancer and treatment efficacy with crizotinib: a multicenter retrospective study. Clin Lung Cancer. 2019;20(2):E171–6. https://doi.org/10.1016/j.cllc.2018.11.007.
Wang SXY, Zhang BM, Wakelee HA, Koontz MZ, Pan MG, Diehn M, et al. Case series of MET exon 14 skipping mutation-positive non-small-cell lung cancers with response to crizotinib and cabozantinib. Anticancer Drugs. 2019;30(5):537–41. https://doi.org/10.1097/cad.0000000000000765.
Wang WX, Wang H, Lu PH, Yu ZY, Xu CW, Zhuang W, et al. Crizotinib with or without an EGFR-TKI in treating EGFR-mutant NSCLC patients with acquired MET amplification after failure of EGFR-TKI therapy: a multicenter retrospective study. J Transl Med. 2019;17:1–9. https://doi.org/10.1186/s12967-019-1803-9.
Wu YL, Yang JC, Kim DW, Lu S, Zhou J, Seto T, et al. Phase II study of crizotinib in East Asian patients with ROS1-positive advanced non-small-cell lung cancer. J Clin Oncol. 2018;36(14):1405–11. https://doi.org/10.1200/jco.2017.75.5587.
Xu H, Zhang Q, Liang L, Li J, Liu Z, Li W, et al. Crizotinib vs platinum-based chemotherapy as first-line treatment for advanced non-small cell lung cancer with different ROS1 fusion variants. Cancer Med. 2020;9(10):3328-36. https://doi.org/10.1002/cam4.2984.
Zeng L, Li Y, Xiao L, Xiong Y, Liu L, Jiang W, et al. Crizotinib presented with promising efficacy but for concomitant mutation in next-generation sequencing-identified ROS1-rearranged non-small-cell lung cancer. Onco Targets Ther. 2018;11:6937–45. https://doi.org/10.2147/ott.s176273.
Zhang L, Jiang T, Zhao C, Li W, Li X, Zhao S, et al. Efficacy of crizotinib and pemetrexed-based chemotherapy in Chinese NSCLC patients with ROS1 rearrangement. Oncotarget. 2016;7(46):75145–54. https://doi.org/10.18632/oncotarget.12612.
Zhu YC, Zhang XG, Lin XP, Wang WX, Li XF, Wu LX, et al. Clinicopathological features and clinical efficacy of crizotinib in Chinese patients with ROS1-positive non-small cell lung cancer. Oncol Lett. 2019;17(3):3466–74. https://doi.org/10.3892/ol.2019.9949.
Shen L, Qiang T, Li Z, Ding D, Yu Y, Lu S. First-line crizotinib versus platinum-pemetrexed chemotherapy in patients with advanced ROS1-rearranged non-small-cell lung cancer. Cancer Med. 2020;9(10):3310-18. https://doi.org/10.1002/cam4.2972.
Shaw AT, Riely GJ, Bang YJ, Kim DW, Camidge DR, Solomon BJ, et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol. 2019;30(7):1121–6. https://doi.org/10.1093/annonc/mdz131.
Hoang T, Myung SK, Pham TT, Park B. Efficacy of crizotinib, ceritinib, and alectinib in ALK-positive non-small cell lung cancer treatment: a meta-analysis of clinical trials. Cancers (Basel). 2020;12(3):526. https://doi.org/10.3390/cancers12030526.
Awad MM, Engelman JA, Shaw AT. Acquired resistance to crizotinib from a mutation in CD74-ROS1. N Engl J Med. 2013;369(12):1173. https://doi.org/10.1056/nejmc1309091.
Davies KD, Mahale S, Astling DP, Aisner DL, Le AT, Hinz TK, et al. Resistance to ROS1 inhibition mediated by EGFR pathway activation in non-small cell lung cancer. PLoS One. 2013;8(12):e82236. https://doi.org/10.1371/journal.pone.0082236.
Zeng L, Li YZ, Xiao LL, Xiong Y, Liu L, Jiang WJ, et al. Crizotinib presented with promising efficacy but for concomitant mutation in next-generation sequencing-identified ROS1-rearranged non-small-cell lung cancer. Oncotargets Ther. 2018;11:6937–45. https://doi.org/10.2147/ott.s176273.
Scagliotti G, von Pawel J, Novello S, Ramlau R, Favaretto A, Barlesi F, et al. Phase III multinational, randomized, double-blind, placebo-controlled study of tivantinib (ARQ 197) plus erlotinib versus erlotinib alone in previously treated patients with locally advanced or metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2015;33(24):2667–74. https://doi.org/10.1200/jco.2014.60.7317.
Yoshioka H, Azuma K, Yamamoto N, Takahashi T, Nishio M, Katakami N, et al. A randomized, double-blind, placebo-controlled, phase III trial of erlotinib with or without a c-Met inhibitor tivantinib (ARQ 197) in Asian patients with previously treated stage IIIB/IV nonsquamous nonsmall-cell lung cancer harboring wild-type epidermal growth factor receptor (ATTENTION study). Ann Oncol. 2015;26(10):2066–72. https://doi.org/10.1093/annonc/mdv288.
Spigel DR, Edelman MJ, O’Byrne K, Paz-Ares L, Shames DS, Yu W, et al. Onartuzumab plus erlotinib versus erlotinib in previously treated stage IIIb or IV NSCLC: Results from the pivotal phase III randomized, multicenter, placebo-controlled METLung (OAM4971g) global trial. J Clin Oncol. 2014;32(15_suppl):8000. https://doi.org/10.1200/jco.2014.32.15_suppl.8000.
Davies KD, Le AT, Theodoro MF, Skokan MC, Aisner DL, Berge EM, et al. Identifying and targeting ROS1 gene fusions in non-small cell lung cancer. Clin Cancer Res. 2012;18(17):4570–9. https://doi.org/10.1158/1078-0432.ccr-12-0550.
Charest A, Wilker EW, McLaughlin ME, Lane K, Gowda R, Coven S, et al. ROS fusion tyrosine kinase activates a SH2 domain-containing phosphatase-2/phosphatidylinositol 3-kinase/mammalian target of rapamycin signaling axis to form glioblastoma in mice. Cancer Res. 2006;66(15):7473–81. https://doi.org/10.1158/0008-5472.can-06-1193.
Jun HJ, Johnson H, Bronson RT, de Feraudy S, White F, Charest A. The oncogenic lung cancer fusion kinase CD74-ROS activates a novel invasiveness pathway through E-Syt1 phosphorylation. Cancer Res. 2012;72(15):3764–74. https://doi.org/10.1158/0008-5472.can-11-3990.
Lu X, Peled N, Greer J, Wu W, Choi P, Berger AH, et al. MET exon 14 mutation encodes an actionable therapeutic target in lung adenocarcinoma. Cancer Res. 2017;77(16):4498–505. https://doi.org/10.1158/0008-5472.can-16-1944.
Schildhaus HU, Schultheis AM, Rüschoff J, Binot E, Merkelbach-Bruse S, Fassunke J, et al. MET amplification status in therapy-naïve adeno- and squamous cell carcinomas of the lung. Clin Cancer Res. 2015;21(4):907–15. https://doi.org/10.1158/1078-0432.ccr-14-0450.
Castiglione R, Alidousty C, Holz B, Wagener S, Baar T, Heydt C, et al. Comparison of the genomic background of MET-altered carcinomas of the lung: biological differences and analogies. Mod Pathol. 2019;32(5):627–38. https://doi.org/10.1038/s41379-018-0182-8.
Schuler M, Berardi R, Lim WT, de Jonge M, Bauer TM, Azaro A, et al. Molecular correlates of response to capmatinib in advanced non-small-cell lung cancer: clinical and biomarker results from a phase I trial. Ann Oncol. 2020;S0923-7534(20)36380-8. https://doi.org/10.1016/j.annonc.2020.03.293.
FDA approves first targeted therapy to treat aggressive form of lung cancer. US Food & Drug Administration. 2020. https://www.fda.gov/news-events/press-announcements/fda-approves-first-targeted-therapy-treat-aggressive-form-lung-cancer. Accessed 15 July 2020.
Wolf J, Seto T, Han JY, Reguart N, Garon EB, Groen HJ, et al. Capmatinib (INC280) in METΔex14-mutated advanced non-small cell lung cancer (NSCLC): efficacy data from the phase II GEOMETRY mono-1 study. J Clin Oncol. 2019;37(15 suppl):9004. https://doi.org/10.1200/jco.2019.37.15_suppl.9004.
Acknowledgements
The authors thank Dr. Michael Magguilli (Oklahoma University Health Sciences Center) for his helpful advice that improved this paper.
Author information
Authors and Affiliations
Contributions
HGV contributed to the study conceptualization, data curation, formal analysis, investigation, methodology, project administration, software, validation, supervision, writing-review, and editing. TQN contributed to the data curation, formal analysis, investigation, software, supervision, writing-review, and editing. HCN contributed to the data curation, formal analysis, investigation, validation, supervision, writing-review, and editing. PTN and ATNH contributed to the data curation, formal analysis, investigation, software, methodology, validation, supervision, writing-review, and editing. LH contributed to the data curation, formal analysis, investigation, software, methodology, project administration, validation, supervision, writing-review, and editing.
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this manuscript.
Conflicts of Interest
HGV, TQN, HCN, PTN, ATNH, and LH have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Rights and permissions
About this article
Cite this article
Vuong, H.G., Nguyen, T.Q., Nguyen, H.C. et al. Efficacy and Safety of Crizotinib in the Treatment of Advanced Non-Small-Cell Lung Cancer with ROS1 Rearrangement or MET Alteration: A Systematic Review and Meta-Analysis. Targ Oncol 15, 589–598 (2020). https://doi.org/10.1007/s11523-020-00745-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-020-00745-7