Abstract
Background
Hepatocellular carcinoma (HCC) represents one of the most lethal cancers worldwide due to therapy resistance and disease recurrence. Tumor relapse following treatment could be driven by the persistence of liver cancer stem-like cells (CSCs). The protein BMI1 is a member of the polycomb epigenetic factors governing cellular self-renewal, proliferation, and stemness maintenance. BMI1 expression also correlates with poor patient survival in various cancer types.
Objective
We aimed to elucidate the extent to which BMI1 can be used as a potential therapeutic target for CSC eradication in HCC.
Methods
We have recently participated in characterizing the first known pharmacological small molecule inhibitor of BMI1. Here, we synthesized a panel of novel BMI1 inhibitors and examined their ability to alter cellular growth and eliminate cancer progenitor/stem-like cells in HCC with different p53 backgrounds.
Results
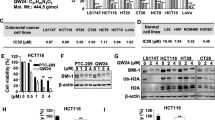
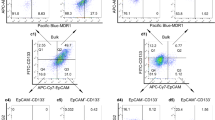
Among various molecules examined, RU-A1 particularly downregulated BMI1 expression, impaired cell viability, reduced cell migration, and sensitized HCC cells to 5-fluorouracil (5-FU) in vitro. Notably, long-term analysis of HCC survival showed that, unlike chemotherapy, RU-A1 effectively reduced CSC content, even as monotherapy. BMI1 inhibition with RU-A1 diminished the number of stem-like cells in vitro more efficiently than the model compound C-209, as demonstrated by clonogenic assays and impairment of CSC marker expression. Furthermore, xenograft assays in zebrafish showed that RU-A1 abrogated tumor growth in vivo.
Conclusions
This study demonstrates the ability to identify agents with the propensity for targeting CSCs in HCC that could be explored as novel treatments in the clinical setting.







Similar content being viewed by others
References
El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–27.
Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362(9399):1907–17.
Yilmaz G, Akyol G, Cakir A, Ilhan M. Investigation of diagnostic utility and expression profiles of stem cell markers (CD133 and CD90) in hepatocellular carcinoma, small cell dysplasia, and cirrhosis. Pathol Res Pract. 2014;210(7):419–25.
Lee TK, Castilho A, Ma S, Ng IO. Liver cancer stem cells: implications for a new therapeutic target. Liver Int. 2009;29(7):955–65.
Yao Z, Mishra L. Cancer stem cells and hepatocellular carcinoma. Cancer Biol Ther. 2009;8(18):1691–8.
Smith LL, Yeung J, Zeisig BB, Popov N, Huijbers I, Barnes J, et al. Functional crosstalk between Bmi1 and MLL/Hoxa9 axis in establishment of normal hematopoietic and leukemic stem cells. Cell Stem Cell. 2011;8(6):649–62.
Chiba T, Miyagi S, Saraya A, Aoki R, Seki A, Morita Y, et al. The polycomb gene product BMI1 contributes to the maintenance of tumor-initiating side population cells in hepatocellular carcinoma. Cancer Res. 2008;68(19):7742–9.
Lukacs RU, Memarzadeh S, Wu H, Witte ON. Bmi-1 is a crucial regulator of prostate stem cell self-renewal and malignant transformation. Cell Stem Cell. 2010;7(6):682–93.
Effendi K, Mori T, Komuta M, Masugi Y, Du W, Sakamoto M. Bmi-1 gene is upregulated in early-stage hepatocellular carcinoma and correlates with ATP-binding cassette transporter B1 expression. Cancer Sci. 2010;101(3):666–72.
Wang H, Pan K, Zhang HK, Weng DS, Zhou J, Li JJ, et al. Increased polycomb-group oncogene Bmi-1 expression correlates with poor prognosis in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2008;134(5):535–41.
Bansal N, Campbell N, Medina D, Dipaola RS, Bertino JR, Sabaawy HE. Targeting Bmi1 in human prostate tumor initiating cells. In: Proceedings of the 101st Annual Meeting of the American Association for Cancer Research. 2010, Philadelphia: AACR: Washington, DC. p. Abstract nr 4279.
Kreso A, van Galen P, Pedley NM, Lima-Fernandes E, Frelin C, Davis T, et al. Self-renewal as a therapeutic target in human colorectal cancer. Nat Med. 2014;20(1):29–36.
Bansal N, Bartucci M, Yusuff S, Davis S, Flaherty K, Huselid E, et al. BMI-1 targeting interferes with patient-derived tumor-initiating cell survival and tumor growth in prostate cancer. Clin Cancer Res. 2016;22(24):6176–91.
Nishida Y, Maeda A, Chachad D, Ishizawa J, Qiu YH, Kornblau SM, et al. Preclinical activity of the novel B-cell-specific Moloney murine leukemia virus integration site 1 inhibitor PTC-209 in acute myeloid leukemia: implications for leukemia therapy. Cancer Sci. 2015;106(12):1705–13.
Mayr C, Wagner A, Loeffelberger M, Bruckner D, Jakab M, Berr F, et al. The BMI1 inhibitor PTC-209 is a potential compound to halt cellular growth in biliary tract cancer cells. Oncotarget. 2016;7(1):745–58.
Bolomsky A, Schlangen K, Schreiner W, Zojer N, Ludwig H. Targeting of BMI-1 with PTC-209 shows potent anti-myeloma activity and impairs the tumour microenvironment. J Hematol Oncol. 2016;9:17.
Liu J, Zhang C, Lin M, Zhu W, Liang Y, Hong X, et al. Glutaminase 2 negatively regulates the PI3K/AKT signaling and shows tumor suppression activity in human hepatocellular carcinoma. Oncotarget. 2014;5(9):2635–47.
Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347(1–2):70–8.
Bansal N, Davis S, Tereshchenko I, Budak-Alpdogan T, Zhong H, Stein MN, et al. Enrichment of human prostate cancer cells with tumor initiating properties in mouse and zebrafish xenografts by differential adhesion. Prostate. 2014;74(2):187–200.
Glide. Available online at http://www.schrodinger.com/docs/2003_1/pdf/firstdiscovery/fd27_technical_notes.pdf . 2003.
Perola E, Walters WP, Charifson PS. A detailed comparison of current docking and scoring methods on systems of pharmaceutical relevance. Proteins. 2004;56(2):235–49.
Hussain SP, Schwank J, Staib F, Wang XW, Harris CC. TP53 mutations and hepatocellular carcinoma: insights into the etiology and pathogenesis of liver cancer. Oncogene. 2007;26(15):2166–76.
Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397(6715):164–8.
Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY, Yang WH, et al. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol. 2010;12(10):982–92.
Grinstein E, Mahotka C. Stem cell divisions controlled by the proto-oncogene BMI-1. J Stem Cells. 2009;4(3):141–6.
Cayrol C, Knibiehler M, Ducommun B. p21 binding to PCNA causes G1 and G2 cell cycle arrest in p53-deficient cells. Oncogene. 1998;16(3):311–20.
Lajtha LG. Stem cell concepts. Differentiation. 1979;14(1–2):23–34.
Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1(5):2315–9.
Sun JH, Luo Q, Liu LL, Song GB. Liver cancer stem cell markers: progression and therapeutic implications. World J Gastroenterol. 2016;22(13):3547–57.
Oishi N, Wang XW. Novel therapeutic strategies for targeting liver cancer stem cells. Int J Biol Sci. 2011;7(5):517–35.
Mihic-Probst D, Kuster A, Kilgus S, Bode-Lesniewska B, Ingold-Heppner B, Leung C, et al. Consistent expression of the stem cell renewal factor BMI-1 in primary and metastatic melanoma. Int J Cancer. 2007;121(8):1764–70.
Glinsky GV, Berezovska O, Glinskii AB. Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J Clin Invest. 2005;115(6):1503–21.
Hayry V, Tynninen O, Haapasalo HK, Wolfer J, Paulus W, Hasselblatt M, et al. Stem cell protein BMI-1 is an independent marker for poor prognosis in oligodendroglial tumours. Neuropathol Appl Neurobiol. 2008;34(5):555–63.
Martin-Padura I, Marighetti P, Agliano A, Colombo F, Larzabal L, Redrado M, et al. Residual dormant cancer stem-cell foci are responsible for tumor relapse after antiangiogenic metronomic therapy in hepatocellular carcinoma xenografts. Lab Investig. 2012;92(7):952–66.
Katoh M, Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res. 2007;13(14):4042–5.
Mehta M, Khan A, Danish S, Haffty BG, Sabaawy HE. Radiosensitization of primary human glioblastoma stem-like cells with low-dose AKT inhibition. Mol Cancer Ther. 2015;14(5):1171–80.
Kim JH, Yoon SY, Jeong SH, Kim SY, Moon SK, Joo JH, et al. Overexpression of Bmi-1 oncoprotein correlates with axillary lymph node metastases in invasive ductal breast cancer. Breast. 2004;13(5):383–8.
Leung C, Lingbeek M, Shakhova O, Liu J, Tanger E, Saremaslani P, et al. Bmi1 is essential for cerebellar development and is overexpressed in human medulloblastomas. Nature. 2004;428(6980):337–41.
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–60.
Berezovska OP, Glinskii AB, Yang Z, Li XM, Hoffman RM, Glinsky GV. Essential role for activation of the Polycomb group (PcG) protein chromatin silencing pathway in metastatic prostate cancer. Cell Cycle. 2006;5(16):1886–901.
Ruan ZP, Xu R, Lv Y, Tian T, Wang WJ, Guo H, et al. Bmi1 knockdown inhibits hepatocarcinogenesis. Int J Oncol. 2013;42(1):261–8.
Sparmann A, van Lohuizen M. Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer. 2006;6(11):846–56.
Fan L, Xu C, Wang C, Tao J, Ho C, Jiang L, et al. Bmi1 is required for hepatic progenitor cell expansion and liver tumor development. PLoS One. 2012;7(9):e46472.
Cao L, Bombard J, Cintron K, Sheedy J, Weetall ML, Davis TW. BMI1 as a novel target for drug discovery in cancer. J Cell Biochem. 2011;112(10):2729–41.
Siddique HR, Saleem M. Role of BMI1, a stem cell factor, in cancer recurrence and chemoresistance: preclinical and clinical evidences. Stem Cells. 2012;30(3):372–8.
Jiang L, Li J, Song L. Bmi-1, stem cells and cancer. Acta Biochim Biophys Sin Shanghai. 2009;41(7):527–34.
Jacobs JJ, Scheijen B, Voncken JW, Kieboom K, Berns A, van Lohuizen M. Bmi-1 collaborates with c-Myc in tumorigenesis by inhibiting c-Myc-induced apoptosis via INK4a/ARF. Genes Dev. 1999;13(20):2678–90.
Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425(6961):962–7.
Dimri GP. What has senescence got to do with cancer? Cancer Cell. 2005;7(6):505–12.
Blackledge NP, Farcas AM, Kondo T, King HW, McGouran JF, Hanssen LL, et al. Variant PRC1 complex-dependent H2A ubiquitylation drives PRC2 recruitment and polycomb domain formation. Cell. 2014;157(6):1445–59.
Fasano CA, Dimos JT, Ivanova NB, Lowry N, Lemischka IR, Temple S. shRNA knockdown of Bmi-1 reveals a critical role for p21-Rb pathway in NSC self-renewal during development. Cell Stem Cell. 2007;1(1):87–99.
Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov. 2009;8(10):806–23.
Teng Y, Xie X, Walker S, White DT, Mumm JS, Cowell JK. Evaluating human cancer cell metastasis in zebrafish. BMC Cancer. 2013;13:453.
Acknowledgements
We thank Leonard Zon (Harvard University) for the Casper zebrafish. We thank members of Dr. David Augeri’s laboratory and core facilities at the Molecular Design and Synthesis laboratory, Rutgers Translational Sciences at Rutgers University for the synthesis, purification and mass spectral analyses of the small molecules utilized in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding for the Study
This project was supported by the Department of Defense Grant (W81XWH-12-1-0249 to H.S.), National Cancer Institute (P30 CA072720 to R.D.) and New Jersey Health Foundation award (Research grant PC-72-16 to H.S.).
Conflict of Interest
The authors declare no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 4339 kb)
Rights and permissions
About this article
Cite this article
Bartucci, M., Hussein, M.S., Huselid, E. et al. Synthesis and Characterization of Novel BMI1 Inhibitors Targeting Cellular Self-Renewal in Hepatocellular Carcinoma. Targ Oncol 12, 449–462 (2017). https://doi.org/10.1007/s11523-017-0501-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-017-0501-x