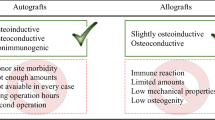
Abstract
Longer-term stability of uncemented femoral stem depends on ossification at bone-implant interface. Although attempts have been made to assess the amount of bone growth using finite element (FE) analysis in combination with a mechanoregulatory algorithm, there has been little research on tissue differentiation patterns on hip stems with proximal macro-textures. The primary goal of this investigation is to qualitatively compare the formation of connective tissues around a femoral implant with/without macro-textures on its proximal surfaces. This study also predicts formation of different tissue phenotypes and their spatio-temporal distribution around a macro-textured femoral stem under routine activities. Results from the study show that non-textured implants (80 to 94%) encourage fibroplasia compared to that in textured implants (71 to 85.38%) under similar routine activity, which might trigger aseptic loosening of implant. Formation of bone was more on medio-lateral sides and towards proximal regions of Gruen zones 2 and 6, which was found to be in line with clinical observations. Fibroplasia was higher under stair climbing (85 to 91%) compared to that under normal walking (71 to 85.38%). This study suggests that stair climbing, although falls under recommended activity, might be detrimental to patient compared to normal walking in the initial rehabilitation period.
Graphical abstract











Similar content being viewed by others
References
Cilla M, Borgiani E, Martínez J, Duda GN, Checa S (2017) Machine learning techniques for the optimization of joint replacements: application to a short-stem hip implant. PLoS One 12(9):e0183755. https://doi.org/10.1371/journal.pone.0183755
Yang L, Kong J, Qiu Z, Shang T, Chen S, Zhao R, Raucci MG, Yang X, Wu Z (2020) Mineralized collagen-modified PMMA cement enhances bone integration and reduces fibrous encapsulation in the treatment of lumbar degenerative disc disease. Regen Biomater 7(2):181–193. https://doi.org/10.1093/rb/rbz044
Ghosh R, Chanda S, Chakraborty D (2020) The influence of macro-textural designs over implant surface on bone on-growth: a computational mechanobiology based study. Comput Biol Med 124:103937. https://doi.org/10.1016/j.compbiomed.2020.103937
Tai CL, Lai PL, Lin WD, Tsai TT, Lee YC, Liu MY, Chen LH (2016) Modification of mechanical properties, polymerization temperature, and handling time of polymethylmethacrylate cement for enhancing applicability in vertebroplasty. Biomed Res Int 2016:7901562. https://doi.org/10.1155/2016/7901562
Puthumanapully PK (2010) Simulation of tissue differentiation in uncemented hip implants based on a mechanoregulatory hypothesis. University of Southampton, School of Engineering Sciences, Doctoral Thesis, pp 221. http://eprints.soton.ac.uk/id/eprint/185117
Chanda S, Mukherjee K, Gupta S, Pratihar DK (2020) A comparative assessment of two designs of hip stem using rule-based simulation of combined osseointegration and remodelling. Proc Inst Mech Eng H 234(1):118–128. https://doi.org/10.1177/0954411919890998
Ghosh R, Chanda S, Chakraborty D (2021) Qualitative predictions of bone growth over optimally designed macro-textured implant surfaces obtained using NN-GA based machine learning framework. Med Eng Phys 95:64–75. https://doi.org/10.1016/j.medengphy.2021.08.002
Ghosh R, Chanda S, Chakraborty D (2021) Influence of sequential opening/closing of interface gaps and texture density on bone growth over macro-textured implant surfaces using FE based mechanoregulatory algorithm. Comput Methods Biomech Biomed Engin 26:1–15. https://doi.org/10.1080/10255842.2021.1994960
Mathai B, Gupta S (2022) Bone ingrowth around an uncemented femoral implant using mechanoregulatory algorithm: a multiscale finite element analysis. J Biomech Eng. 144(2):021004. https://doi.org/10.1115/1.4052227
Causey GC, Picha GJ, Price J, Pelletier MH, Wang T, Walsh WR (2021) In-Vivo response to a novel pillared surface morphology for osseointegration in an ovine model. J Mech Behav Biomed Mater 119:104462. https://doi.org/10.1016/j.jmbbm.2021.104462
Bergmann G, Graichen F, Rohlmann A (1995) Is staircase walking a risk for the fixation of hip implants? J Biomech 28(5):535–53. https://doi.org/10.1016/0021-9290(94)00105-d
Kurtz S, Ong K, Lau E, Mowat F, Halpern M (2007) Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 89(4):780–5. https://doi.org/10.2106/JBJS.F.00222
Bhattacharyya T, Chang D, Meigs JB, Estok DM 2nd, Malchau H (2007) Mortality after periprosthetic fracture of the femur. J Bone Joint Surg Am 89(12):2658–62. https://doi.org/10.2106/JBJS.F.01538
Folgado J, Fernandes PR, Jacobs CR, Pellegrini VD Jr (2009) Influence of femoral stem geometry, material and extent of porous coating on bone ingrowth and atrophy in cementless total hip arthroplasty: an iterative finite element model. Comput Methods Biomech Biomed Engin 12(2):135–45. https://doi.org/10.1080/10255840903081123
Tarala M, Janssen D, Verdonschot N (2011) Balancing incompatible endoprosthetic design goals: a combined ingrowth and bone remodeling simulation. Med Eng Phys 33(3):374–80. https://doi.org/10.1016/j.medengphy.2010.11.005
Mukherjee K, Gupta S (2016) Bone ingrowth around porous-coated acetabular implant: a three-dimensional finite element study using mechanoregulatory algorithm. Biomech Model Mechanobiol 15(2):389–403. https://doi.org/10.1007/s10237-015-0696-7
Claes LE, Heigele CA (1999) Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing. J Biomech 32(3):255–66. https://doi.org/10.1016/s0021-9290(98)00153-5
Viceconti M, Bellingeri L, Cristofolini L, Toni A (1998) A comparative study on different methods of automatic mesh generation of human femurs. Med Eng Phys 20(1):1–10. https://doi.org/10.1016/s1350-4533(97)00049-0
Fraldi M, Esposito L, Perrella G, Cutolo A, Cowin SC (2010) Topological optimization in hip prosthesis design. Biomech Model Mechanobiol 9(4):389–402. https://doi.org/10.1007/s10237-009-0183-0
Isaksson H, van Donkelaar CC, Huiskes R, Ito K (2006) Corroboration of mechanoregulatory algorithms for tissue differentiation during fracture healing: Comparison with in vivo results. J Orthop Res 24(5):898–907. https://doi.org/10.1002/jor.20118
Tarlochan F, Mehboob H, Mehboob A, Chang SH (2018) Influence of functionally graded pores on bone ingrowth in cementless hip prosthesis: a finite element study using mechano-regulatory algorithm. Biomech Model Mechanobiol 17(3):701–716. https://doi.org/10.1007/s10237-017-0987-2
Mehboob H, Ahmad F, Tarlochan F, Mehboob A, Chang SH (2020) A comprehensive analysis of bio-inspired design of femoral stem on primary and secondary stabilities using mechanoregulatory algorithm. Biomech Model Mechanobiol 19(6):2213–2226. https://doi.org/10.1007/s10237-020-01334-3
Mohandes Y, Tahani M, Rouhi G, Tahami M (2021) A mechanobiological approach to find the optimal thickness for the locking compression plate: finite element investigations. Proc Inst Mech Eng H 235(4):408–418. https://doi.org/10.1177/0954411920985757
Taddei F, Schileo E, Helgason B, Cristofolini L, Viceconti M (2007) The material mapping strategy influences the accuracy of CT-based finite element models of bones: an evaluation against experimental measurements. Med Eng Phys 29(9):973–9. https://doi.org/10.1016/j.medengphy.2006.10.014
Heller MO, Bergmann G, Kassi JP, Claes L, Haas NP, Duda GN (2005) Determination of muscle loading at the hip joint for use in pre-clinical testing. J Biomech 38(5):1155–63. https://doi.org/10.1016/j.jbiomech.2004.05.022
Pedersen DR, Brand RA, Davy DT (1997) Pelvic muscle and acetabular contact forces during gait. J Biomech 30(9):959–65. https://doi.org/10.1016/s0021-9290(97)00041-9
Andreykiv A, van Keulen F, Prendergast PJ (2008) Computational mechanobiology to study the effect of surface geometry on peri-implant tissue differentiation. J Biomech Eng 130(5):051015. https://doi.org/10.1115/1.2970057
Barry FP (2003) Biology and clinical applications of mesenchymal stem cells. Birth Defects Res C Embryo Today 69(3):250–6. https://doi.org/10.1002/bdrc.10021
Isaksson H, Wilson W, van Donkelaar CC, Huiskes R, Ito K (2006) Comparison of biophysical stimuli for mechano-regulation of tissue differentiation during fracture healing. J Biomech 39(8):1507–1516. https://doi.org/10.1016/j.jbiomech.2005.01.037
Puthumanapully PK, Browne M (2011) Tissue differentiation around a short stemmed metaphyseal loading implant employing a modified mechanoregulatory algorithm: a finite element study. J Orthop Res 29(5):787–94. https://doi.org/10.1002/jor.21305
Tan F, Wang C, Yang C, Huang Y, Fan Y (2017) Biomechanical effects of various bone-implant interfaces on the stability of orthodontic miniscrews: a finite element study. J Healthc Eng 2017:7495606. https://doi.org/10.1155/2017/7495606
Giori NJ, Beaupré GS, Carter DR (1993) Cellular shape and pressure may mediate mechanical control of tissue composition in tendons. J Orthop Res 11(4):581–91. https://doi.org/10.1002/jor.1100110413
Nowlan NC, Murphy P, Prendergast PJ (2007) Mechanobiology of embryonic limb development. Ann N Y Acad Sci 1101:389–411. https://doi.org/10.1196/annals.1389.003
Breeland G, Sinkler MA, Menezes RG (2021) Embryology, bone ossification. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing
Stolk J, Verdonschot N, Huiskes R (2002) Stair climbing is more detrimental to the cement in hip replacement than walking. Clin Orthop Relat Res 405:294–305. https://doi.org/10.1097/00003086-200212000-00037
Kuzyk PR, Schemitsch EH (2011) The basic science of peri-implant bone healing. Indian J Orthop. 45(2):108–15. https://doi.org/10.4103/0019-5413.77129
Burke DP, Kelly DJ (2012) Substrate stiffness and oxygen as regulators of stem cell differentiation during skeletal tissue regeneration: a mechanobiological model. PLoS One 7(7):e40737. https://doi.org/10.1371/journal.pone.0040737
Liu X, Niebur GL (2008) Bone ingrowth into a porous coated implant predicted by a mechano-regulatory tissue differentiation algorithm. Biomech Model Mechanobiol 7(4):335–44. https://doi.org/10.1007/s10237-007-0100-3
Sennerby L, Thomsen P, Ericson LE (1993) Early tissue response to titanium implants inserted in rabbit cortical bone. J Mater Sci: Mater Med 4:240–250. https://doi.org/10.1007/BF00122275
Choi JY, Sim JH, Yeo IL (2017) Characteristics of contact and distance osteogenesis around modified implant surfaces in rabbit tibiae. J Periodontal Implant Sci 47(3):182–192. https://doi.org/10.5051/jpis.2017.47.3.182
Berglundh T, Abrahamsson I, Lang NP, Lindhe J (2003) De novo alveolar bone formation adjacent to endosseous implants. Clin Oral Implants Res 14(3):251–62. https://doi.org/10.1034/j.1600-0501.2003.00972.x
Carter DR, Beaupré GS, Giori NJ, Helms JA (1998) Mechanobiology of skeletal regeneration. Clin Orthop Relat Res 355 Suppl:S41-55. https://doi.org/10.1097/00003086-199810001-00006
Santori N, Lucidi M, Santori FS (2006) Proximal load transfer with a stemless uncemented femoral implant. J Orthop Traumatol 7(3):154–160. https://doi.org/10.1007/s10195-006-0141-x
Santori N, Albanese CV, Learmonth ID, Santori FS (2006) Bone preservation with a conservative metaphyseal loading implant. Hip Int 16(Suppl 3):16–21. https://doi.org/10.5301/hip.2009.3327
Fernandes PR, Folgado J, Jacobs C, Pellegrini V (2002) A contact model with ingrowth control for bone remodelling around cementless stems. J Biomech 35(2):167–76. https://doi.org/10.1016/s0021-9290(01)00204-4
Andreykiv A, Janssen D, Nelissen RG, Valstar ER (2012) On stabilization of loosened hip stems via cement injection into osteolytic cavities. Clin Biomech (Bristol, Avon) 27(8):807–12. https://doi.org/10.1016/j.clinbiomech.2012.04.004
Huiskes R, Weinans H, Grootenboer HJ, Dalstra M, Fudala B, Slooff TJ (1987) Adaptive bone-remodeling theory applied to prosthetic-design analysis. J Biomech 20(11–12):1135–1150. https://doi.org/10.1016/0021-9290(87)90030-3
Chennimalai Kumar N, Dantzig JA, Jasiuk IM, Robling AG, Turner CH (2010) Numerical modeling of long bone adaptation due to mechanical loading: correlation with experiments. Ann Biomed Eng 38(3):594–604. https://doi.org/10.1007/s10439-009-9861-4
Avval PT, Samiezadeh S, Bougherara H (2016) Long-term response of femoral density to hip implant and bone fracture plate: computational study using a mechano-biochemical model. Med Eng Phys 38(2):171–80. https://doi.org/10.1016/j.medengphy.2015.11.013
Isaksson H, van Donkelaar CC, Huiskes R, Ito K (2008) A mechano-regulatory bone-healing model incorporating cell-phenotype specific activity. J Theor Biol 252(2):230–46. https://doi.org/10.1016/j.jtbi.2008.01.030
Mukherjee K, Gupta S (2017) Mechanobiological simulations of peri-acetabular bone ingrowth: a comparative analysis of cell-phenotype specific and phenomenological algorithms. Med Biol Eng Comput 55(3):449–465. https://doi.org/10.1007/s11517-016-1528-3
Andreykiv A, van Keulen F, Prendergast PJ (2008) Simulation of fracture healing incorporating mechanoregulation of tissue differentiation and dispersal/proliferation of cells. Biomech Model Mechanobiol 7(6):443–61. https://doi.org/10.1007/s10237-007-0108-8
Gómez-Benito MJ, García-Aznar JM, Kuiper JH, Doblaré M (2005) Influence of fracture gap size on the pattern of long bone healing: a computational study. J Theor Biol 235(1):105–19. https://doi.org/10.1016/j.jtbi.2004.12.023
Ambard D, Swider P (2006) A predictive mechano-biological model of the bone-implant healing. Eur J Mech A/Solids 25(6):927–37. https://doi.org/10.1016/j.euromechsol.2006.02.006
Ghosh R, Chanda S, Chakraborty D (2022) Application of finite element analysis to tissue differentiation and bone remodelling approaches and their use in design optimization of orthopaedic implants: a review. Int J Numer Method Biomed Eng 38(10). https://doi.org/10.1002/cnm.3637
Acknowledgements
The authors would like to acknowledge Agartala Government Medical College, Agartala, India for providing with the CT scan data set. The authors would also like to acknowledge the computational facilities available at the Composite Structures and Fracture Mechanics Laboratory, Department of Mechanical Engineering, Indian Institute of Technology Guwahati, India, which has helped to carry out the research study. The study was partially funded by SPARC, MHRD, Government of India (Project ID: SPARC/P705).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghosh, R., Hazra, A., Chanda, S. et al. Computational assessment of growth of connective tissues around textured hip stem subjected to daily activities after THA. Med Biol Eng Comput 61, 525–540 (2023). https://doi.org/10.1007/s11517-022-02729-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-022-02729-3