Abstract
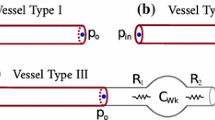
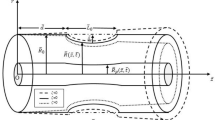
Traditionally, the arteries in mammals are viewed as the tubes with elastic wall, whose elasticity could be slowly (during a minute) tuned to change its diameter thereby regulating regional blood supply. Recent findings showed that an artery is a much more sophisticated organ, which can change elasticity of vascular wall within a fraction of a second during a cardiac cycle due to activation of its smooth muscles manifested by generation of arterial action potentials. The rapid variations in elasticity of vascular wall resulted in three basic modes of arterial pulsing: passive, active, and intermediate. The latter is characterized by counteraction of dilation force of arterial pressure and the contractile one of smooth muscle in arterial wall, which can result in seemingly “rigid” artery of constant diameter. The prevalence of any of these forces results in active or passive pulsing modes. Existence of various pulsing modes raises the question of their effect on the main function of blood vessels, i.e., the transport of blood. The aim of this study is to assess the effect of various modes of arterial pulsing on hydraulic impedance of major arteries. The linearized Navier–Stokes equation was employed to develop a model of pulsatile flow of viscous incompressible fluid at small velocity via a conduit artery with the walls of variable or constant elasticity. An essential feature of the developed model is the shape of variable pressure drop applied to the ends of arterial segment, which simulates the real changes in arterial pressure during the heartbeat. Here, it is modeled by periodic (systolic) positive bell-shaped impulses with maxima corresponding to systolic arterial pressure, while the minimal plateau level refers to diastolic arterial pressure. The model assesses the changes in arterial hydraulic impedance during a cardiac cycle relatively to the stable level corresponding to constant blood flow driven by persistent pressure drop. Within intermediate variety of pulsing modes between the active and passive ones, the approximation of rigid arterial segment with infinite elasticity of arterial wall showed that hydraulic impedance in rigid artery is not constant due to inertial properties of the flowing blood. In passive pulsing mode characterized with constant elasticity of arterial wall, the diameter of artery changes in parallel with systolic pressure applied to the ends of arterial segment. At this, the overall change of hydraulic resistance is negative. In active pulsing mode, elasticity of arterial wall varies at different phase shifts relative to arterial pressure due to periodic contractions and relaxation of the smooth muscles in arterial wall. An important feature of active mode is possibility to decrease the hydraulic impedance during the front of arterial pressure. Various experimental modes of artery pulsing can be mathematically simulated. The passive and active modes of pulsing as well as a broad variety of intermediate pulsing modes with various phase shifts between arterial pressure and its diameter result in potency of the arteries to tune its performance in order to meet the regional circulatory requirements. The model showed that active arterial pulsing can diminish the arterial hydraulic impedance and contribute to the work needed for circulation thereby helping the pumping action of the heart.
Graphical abstract






Similar content being viewed by others
Abbreviations
- V :
-
Blood velocity
- P :
-
Pressure drop
- ν :
-
Blood kinematic viscosity
- η :
-
Dynamic viscosity
- L :
-
Arterial length
- \(\delta {P}_0\) :
-
Constant pressure drop
- T :
-
Period of heartbeats
- a :
-
Arterial radius
- Q(t):
-
Total volume flow rate
- R(t):
-
Arterial hydraulic impedance
- R 0 :
-
Stationary hydraulic impedance
- δp(t):
-
Variable pressure drop
- C :
-
Constant elasticity of arterial wall
- \(C^{\prime}\) :
-
Variable elasticity of arterial wall
- S :
-
Cross-section area of a tube at a given site
- S 0 :
-
Arterial cross-section area at zero excess pressure
- E :
-
Effective Young’s modulus of the tube wall
- h :
-
Wall thickness
- \(\delta^{\left( 2 \right)} V\left( t \right)\) :
-
Additional velocity
- δ :
-
Phase shift
- A 0 :
-
Work performed by the heart during one beat
- A 1,2,3 :
-
Work of the heart related to variable part of pressure
References
Pedley TJ (2012) The fluid mechanics of large blood vessels. Cambridge University Press, Cambridge
Grimnes S, Martinsen O (2014) Bioimpedance and bioelectricity basics, 3rd edn. Academic Press, New York
Caro CG, Pedley TJ, Schroter RC, Seed WA (2012) The mechanics of the circulation, 2nd edn. Cambridge University Press, Cambridge
Revenko SV, Tikhomirova LN, Nesterov AV, Tarakanov IA (2018) Bimodal electric properties of rat major artery segment in situ. Bull Exp Biol Med 164(6):701–706
Revenko SV, Tikhomirova LN, Gavrilov IYu, Tarakanov IA (2019) Effect of alternating electric current on pulsation mode of rat major arteries in situ. Bull Exp Biol Med 167(3):305–310
Tarakanov IA, Tikhomirova LN, Gavrilov IYu, Revenko SV (2020) Relationship between passive and active pulsations of rat artery as vascular extension of Frank-Starling law. Bull Exp Biol Med 170(12):698–703 ((in Russian))
Misbah Ch (2012) Vesicles, capsules and red blood cells under flow. J Phys Conf Ser 392:012005
Vlahovska PM, Podgorski T, Misbah Ch (2009) Vesicles and red blood cells in flow: from individual dynamics to rheology. C R Phys 10(8):775–789
Markosyan TG, Alyaev YuG, Glybochko PV, Kirpatovsky VI, Mudraya IS, Nikitin SS, Popov EG, Revenko SV (2011) Rhythmic oscillations of human penile bioimpedance in healthy individuals and in patients with vascular erectile dysfunction. Bull. Exp. Biol. Med. 151(4):405–410
Mano T, Ivase S, Toma S (2006) Microneurography as a tool in clinical neurophysiology to investigate peripheral neural traffic in humans. Clin Neurophysiol 117(11):2357–2384
Jünemann KP, Scheepe J, Persson-Jünemann C, Schmidt P, Abel K, Zwick A, Tschada R, Alken P (1994) Basic experimental studies on corpus cavernosum electromyography and smooth-muscle electromyography of the urinary bladder. World J Urol 12(5):266–273
Davis MJ, Hill MA, Kuo L (2008) Local regulation of microvascular perfusion. In: Tuma RF, Duran WN, Ley K (eds) Handbook of physiology: microcirculation, 2nd edn. Academic Press, San Diego, pp 161–284
Volobuev AN (1995) Fluid flow in tubes with elastic walls. Phys Usp 38(2):169–178
Womersley J (1955) Method for the calculation of velocity, rate of flow and viscous drag in arteries when the pressure gradient is known. J Physique 127(3):553–563
Nichols WW, O'Rourke MF (2005) McDonald’s blood flow in arteries, 5th edn. Hodder-Arnold, London (England)
Kedrov AA (1941) A novel method to determine pulsatile oscillations in blood filling of vasculature in various regions of human body. Clin Med 19(1):71–80
Kaoui B, Biros G, Misbah Ch (2009) Why do red blood cells have asymmetric shapes even in a symmetric flow? Phys Rev Lett 103:188101
Müller LO, Toro EF (2014) A global multiscale mathematical model for the human circulation with emphasis on the venous system. Int J Numer Method Biomed Eng 30(7):681–725
Mabotuwana TDS, Cheng LK, Pullan AJ (2007) A model of blood flow in the mesenteric arterial system. Biomed Eng Online 6(1):17
Munakata M (2014) Brachial-ankle pulse wave velocity in the measurement of arterial stiffness: recent evidence and clinical applications. Curr Hypertens Rev 10(1):49–57
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Belousov, Y.M., Krainov, V.P. & Revenko, S.V. Hydrodynamic model of blood flow in major arteries pulsing in various modes. Med Biol Eng Comput 59, 1785–1794 (2021). https://doi.org/10.1007/s11517-021-02413-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-021-02413-y