Abstract
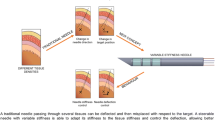
Needles are tools that are used daily during minimally invasive procedures. During the insertions, needles may be affected by deformations which may threaten the success of the procedure. To tackle this problem, needles with embedded strain sensors have been developed and associated with navigation systems. The localization of the needle in the tissues is then obtained in real time by reconstruction from the strain measurements, allowing the physician to optimize its gesture. As the number of strain sensors embedded is limited in number, their positions on the needle have a great impact on the accuracy of the shape reconstruction. The main contribution of this paper is a novel strain sensor positioning method to improve the reconstruction accuracy. A notable feature of our method is the use of experimental needle insertion data, which increases the relevancy of the resulting sensor optimal locations. To the best of the author’s knowledge, no experimentally based needle sensor positioning method has been presented yet. Reconstruction validations from clinical data show that the localization accuracy of the needle tip is improved by almost 40% with optimal locations compared with equidistant locations when reconstructing with two sensor triplets or more.

Improvement of the reconstruction accuracy of a deformed needle shape by using experimental data to position strain sensors







Similar content being viewed by others
References
Abayazid M, Kemp M, Misra S (2013) 3d flexible needle steering in soft-tissue phantoms using fiber Bragg grating sensors. In: Proceedings of IEEE international conference on robotics and automation (ICRA), pp 5843–5849. https://doi.org/10.1109/ICRA.2013.6631418
Adams R, Bischof L (1994) Seeded region growing. IEEE Trans Pattern Anal Mach Intell 16(6):641–647
Bishop RL (1975) There is more than one way to frame a curve. Amer Math Mon 82(3):246–251. http://www.jstor.org/stable/2319846
Bonvilain A, Gangneron M (2016) Characterization of strain microgauges for the monitoring of the deformations of a medical needle during its insertion in human tissues. Microsyst Technol 22(3):551–556. https://doi.org/10.1007/s00542-015-2588-2
Bonvilain A, Zanardelli L, Carriquiry A (2018) Piezoresistif microsensors for an instrumented medical needle for its real time monitoring in a microlocalization tool. Microsyst Technol 24(7):3161–3167. https://doi.org/10.1007/s00542-018-3814-5
de Boor C (1978) A practical guide to splines. Springer, New York
Brett PN, Parker T, Harrison AJ, Thomas TA, Carr A (1997) Simulation of resistance forces acting on surgical needles. Proceedings of the Institution of Mechanical Engineers. Part H: J Eng Med 211(4):335–347
Byrd RH, Nocedal J, Waltz RA (2006) Knitro: an integrated package for nonlinear optimization. In: Large-scale nonlinear optimization. Springer, pp 35–59
Cosserat E, Cosserat F et al (1909) Théorie des corps déformables
Craven P, Wahba G (1978) Smoothing noisy data with spline functions. Numer Math 31(4):377–403. https://doi.org/10.1007/BF01404567
Fouard C, Deram A, Keraval Y, Promayon E (2012) CamiTK: a modular framework integrating visualization, image processing and biomechanical modeling. In: Payan Y (ed) Soft Tissue Biomechanical Modeling for Computer Assisted Surgery, pp 323–354
Guggenheimer H (1989) Computing frames along a trajectory. Comput Aided Geom Des 6(1):77–78
Hairer E, Wanner G, Lubich C (2006) Geometric Numerical Integration. Structure-preserving Algorithms for Ordinary Differential Equations, 2 edn
Haron H, Rehman A, Adi D, Lim S, Saba T (2012) Parameterization method on b-spline curve. Mathematical Problems in Engineering 2012
van der Heiden MS, Henken K, Chen LK, van den Bosch BG, van den Braber R, Dankelman J, van den Dobbelsteen J (2012) Accurate and efficient fiber optical shape sensor for mri compatible minimally invasive instruments. https://doi.org/10.1117/12.981141
Henken K, Gerwen DV, Dankelman J, Dobbelsteen JVD (2012) Accuracy of needle position measurements using fiber Bragg gratings. Minim Invasive Ther Allied Technol 21(6):408–414. https://doi.org/10.3109/13645706.2012.666251
Henken K, Dankelman J, van den Dobbelsteen J, Cheng LK, van der Heiden MS (2014) Error analysis of fbg-based shape sensors for medical needle tracking. 19(5):1523–1531. https://doi.org/10.1109/TMECH.2013.2287764
Hocking G, Hebard S, Mitchell CH (2011) A review of the benefits and pitfalls of phantoms in ultrasound-guided regional anesthesia. Region Anesth Pain Med 36(2):162–170
Jiang S, Li P, Yu Y, Liu J, Yang Z (2014) Experimental study of needletissue interaction forces: Effect of needle geometries, insertion methods and tissue characteristics. J Biomech 47(13):3344–3353. https://doi.org/10.1016/j.jbiomech.2014.08.007
Kim B, Ha J, Park FC, Dupont PE (2014) Optimizing curvature sensor placement for fast, accurate shape sensing of continuum robots. In: 2014 IEEE International conference on robotics and automation (ICRA), pp 5374–5379. https://doi.org/10.1109/ICRA.2014.6907649
Kirchhoff G (1859) Uber das gleichgewicht und die bewegung eines unendlich dunnen elastischen stabes. J Reine Angew Math 56:285–313
Le Digabel S (2011) Algorithm 909: Nomad: Nonlinear optimization with the mads algorithm. ACM Trans Math Softw 37(4):44:1–44:15. https://doi.org/10.1145/1916461.1916468
Lee E (1989) Choosing nodes in parametric curve interpolation. Comput-Aided Des 21(6):363–370. https://doi.org/10.1016/0010-4485(89)90003-1
Love AEH (1906) A treatise on the mathematical theory of elasticity, 2 edn
Magnus W (1954) On the exponential solution of differential equations for a linear operator. Commun Pur Appl Math 7(4):649–673. https://doi.org/10.1002/cpa.3160070404
Mahoney AW, Bruns TL, Swaney PJ, Webster RJ (2016) On the inseparable nature of sensor selection, sensor placement, and state estimation for continuum robots or where to put your sensors and how to use them. In: 2016 IEEE international conference on robotics and automation (ICRA). IEEE, pp 4472– 4478
Monasa F, Lewis G (1983) Large deflections of point loaded cantilevers with nonlinear behaviour. ZAMP Z Angew Math Phys 34(1):124–130. https://doi.org/10.1007/bf00962621
Moon H, Jeong J, Kang S, Kim K, Song YW, Kim J (2014) Fiber-bragg-grating-based ultrathin shape sensors displaying single-channel sweeping for minimally invasive surgery. Opt Lasers Eng 59:50–55. https://doi.org/10.1016/j.optlaseng.2014.03.005
Moreira P, Misra S (2015) Biomechanics-based curvature estimation for ultrasound-guided flexible needle steering in biological tissues. Ann Biomed Eng 43(8):1716–1726. https://doi.org/10.1007/s10439-014-1203-5
Ng KW, Goh JQ, Foo SL, Ting PH, Lee TK (2013) Needle insertion forces studies for optimal surgical modeling. International Journal of Bioscience. Bioch Bioinforma 3(3):187
Park YL, Elayaperumal S, Daniel B, Ryu SC, Shin M, Savall J, Black R, Moslehi B, Cutkosky M (2010) Real-time estimation of 3-d needle shape and deflection for mri-guided interventions. 15(6):906–915. https://doi.org/10.1109/TMECH.2010.2080360
Podder TK, Clark DP, Sherman J (2005) Effects of tip geometry of surgical needle an assessment of force and deflection. Third European medical and biological engineering conference, Prague, Czech Republic, pp 1641–1644
Ragozin DL (1983) Error bounds for derivative estimates based on spline smoothing of exact or noisy data. J Approx Theory 37(4):335–355. https://doi.org/10.1016/0021-9045(83)90042-4
Rao CK, Deshpande AP (2010) Modelling of engineering materials. Ane Books Pvt Ltd
Reissner E (1973) On one-dimensional large-displacement finite-strain beam theory. Stud Appl Math 52 (2):87–95
Rice J, Rosenblatt M (1981) Integrated mean squared error of a smoothing spline. J Approx Theory 33 (4):353–369. https://doi.org/10.1016/0021-9045(81)90066-6
Robert AL, Chagnon G, Bricault I, Cinquin P, Moreau-Gaudry A (2013) A generic three-dimensional static force distribution basis for a medical needle inserted into soft tissue. J Mech Behav Biomed Mater 28:156–170. https://doi.org/10.1016/j.jmbbm.2013.07.023
Rocco Furferi Lapo Governi MPYV (2011) From unordered point cloud to weighted b-spline -. a novel pca-based method - Applications of Mathematics and Computer Engineering
Roesthuis R, Kemp M, van den Dobbelsteen J, Misra S (2014) Three-dimensional needle shape reconstruction using an array of fiber Bragg grating sensors 19(4):1115–1126. https://doi.org/10.1109/TMECH.2013.2269836
Roesthuis RJ, Janssen S, Misra S (2013) On using an array of fiber bragg grating sensors for closed-loop control of flexible minimally invasive surgical instruments. In: 2013 IEEE/RSJ International conference on intelligent robots and systems, pp 2545–2551. https://doi.org/10.1109/IROS.2013.6696715
Rouchy R, Moreau-Gaudry A, Chipon E, Aubry S, Pazart L, Lapuyade B, Durand M, Hajjam M, Pottier S, Renard B, Logier R, Orry X, Cherifi A, Quehen E, Kervio G, Favelle O, Patat F, De Kerviler E, Hughes C, Medici M, Ghelfi J, Mounier A, Bricault I (2017) Evaluation of the clinical benefit of an electromagnetic navigation system for ct-guided interventional radiology procedures in the thoraco-abdominal region compared with conventional ct guidance (ctnav ii): study protocol for a randomised controlled trial. Trials 18(1):306. https://doi.org/10.1186/s13063-017-2049-6
Seifabadi R, Gomez EE, Aalamifar F, Fichtinger G, Iordachita I (2013) Real-time tracking of a bevel-tip needle with varying insertion depth: Toward teleoperated mri-guided needle steering. In: 2013 IEEE/RSJ International conference on intelligent robots and systems, pp 469–476. https://doi.org/10.1109/IROS.2013.6696393
Simo J, Fox D (1989) On a stress resultant geometrically exact shell model. part i: Formulation and optimal parameterization. Comput Methods Appl Mech Eng 72(3):267–304. https://doi.org/10.1016/0045-7825(89)90002-9
Sultan SF, Shorten G, Iohom G (2013) Simulators for training in ultrasound guided procedures. Med Ultrasonogr 15(2):125–131
Todd MD, Stull CJ, Dickerson M (2013) A local material basis solution approach to reconstructing the three-dimensional displacement of rod-like structures from strain measurements. Journal of Applied Mechanics 80 (4):041028
Wan G, Wei Z, Gardi L, Downey DB, Fenster A (2005) Brachytherapy needle deflection evaluation and correction. Med Phys 32(4):902–909. https://doi.org/10.1118/1.1871372
Wang W, Jüttler B, Zheng D, Liu Y (2008) Computation of rotation minimizing frames. ACM Trans Graph (TOG) 27(1):2
Whittaker S, Lethbridge G, Kim C, Keon Cohen Z, Ng I An ultrasound needle insertion guide in a porcine phantom model. Anaesthesia 68(8):826–829. https://doi.org/10.1111/anae.12262
Wood GA (1982) Data smoothing and differentiation procedures in biomechanics. Exerc Sport Sci Rev 10 (1):308–362
Xu R, Yurkewich A, Patel RV (2016) Shape sensing for torsionally compliant concentric-tube robots. https://doi.org/10.1117/12.2213128
Acknowledgments
This work is part of the project GAME-D, financed by the French National Agency for Research (ref: ANR-12-TECS-0019) and supported by Laboratory of Excellence CAMI (ref: ANR-11-LABX-0004-01).
The authors would like to thank Benjamin Spencer and Cecilia Hughes for their English reviews and corrections.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schaefer, PL., Chagnon, G. & Moreau-Gaudry, A. Optimized needle shape reconstruction using experimentally based strain sensors positioning. Med Biol Eng Comput 57, 1901–1916 (2019). https://doi.org/10.1007/s11517-019-02001-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-019-02001-1