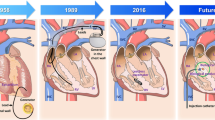
Abstract
Cardiac rhythm-associated disorders are caused by mal-functions of impulse generation and conduction. Present therapies for the impulse generation span a wide array of approaches but remain largely palliative. The progress in the understanding of the biology of the diseases with related biological tools beckons for new approaches to provide better alternatives to the present routine. Here, we review the current state of the art in gene- and cell-based approaches to correct cardiac rhythm disturbances. These include genetic suppression of an ionic current, stem cell therapies, adult somatic cell-fusion approach, novel synthetic pacemaker channel, and creating a self-contained pacemaker activity in non-excitable cells. We then conclude by discussing advantages and disadvantages of the new possibilities.





Similar content being viewed by others
References
Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM et al (2003) Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 425(6961):968–973
Anversa P, Kajstura J, Leri A, Bolli R (2006) Life, death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation 113(11):1451–1453
Azene EM, Xue T, Marban E, Tomaselli GF, Li RA (2005) Non-equilibrium behavior of HCN channels: insights into the role of HCN channels in native and engineered pacemakers. Cardiovasc Res 67(2):263–273
Barr L, Dewey MM, Berger W (1965) Propagation of action potentials and the structure of the nexus in cardiac muscle. J Gen Physiol 48:797–823
Beltrami AP, Barlucchi L, Torella D et al (2003) Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114(6):763–776
Bernstein AD, Parsonnet V (2001) Survey of cardiac pacing and implanted defibrillator practice patterns in the United States in 1997. Pacing Clin Electrophysiol 24(5):842–855
Blaese RM, Culver KW, Miller AD et al (1995) T lymphocyte-directed gene therapy for ADA- SCID: initial trial results after 4 years. Science 270(5235):475–480
Brewster AL, Bernard JA, Gall CM, Baram TZ (2005) Formation of heteromeric hyperpolarization-activated cyclic nucleotide-gated (HCN) channels in the hippocampus is regulated by developmental seizures. Neurobiol Dis 19(1–2):200–207
Brooks CM, Lu H-h (1972) The sinoatrial pacemaker of the heart. Charles C. Thomas, Springfield
Brown HF, McNaughton PA, Noble D, Noble SJ (1975) Adrenergic control of cardian pacemaker currents. Philos Trans R Soc Lond B Biol Sci 270(908):527–537
Brown HF, Kimura J, Noble D, Noble SJ, Taupignon A (1984) The ionic currents underlying pacemaker activity in rabbit sino-atrial node: experimental results and computer simulations. Proc R Soc Lond B Biol Sci 222(1228):329–347
Campbell DL, Giles WR, Shibata EF (1988) Ion transfer characteristics of the calcium current in bull-frog atrial myocytes. J Physiol 403:239–266
Cerbai E, Pino R, Porciatti F et al (1997) Characterization of the hyperpolarization-activated current, I(f), in ventricular myocytes from human failing heart. Circulation 95(3):568–571
Cho HC, Kashiwakura Y, Marban E (2005) Creation of a biological pacemaker by cell fusion. Circulation 112(17):II-307
Cho HC, Kashiwakura Y, Marban E (2005) Conversion of non-excitable cells to self-contained biological pacemakers. Circulation 112(17):II-307
Cho HC, Smith R, Abraham M, Messina E, Giacomello A, Marban E (2005) Electrophysiology of human and porcine adult cardiac stem cells isolated from endomyocardial biopsies. Heart Rhythm 2(5):S45
Coplen SE, Antman EM, Berlin JA, Hewitt P, Chalmers TC (1990) Efficacy and safety of quinidine therapy for maintenance of sinus rhythm after cardioversion. A meta-analysis of randomized control trials. Circulation 82(4):1106–1116
DiFrancesco D (1995) The pacemaker current (I(f)) plays an important role in regulating SA node pacemaker activity. Cardiovasc Res 30(2):307–308
Echt DS, Liebson PR, Mitchell LB et al (1991) Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The cardiac arrhythmia suppression trial. N Engl J Med 324(12):781–788
Falk RH (2001) Atrial fibrillation. N Engl J Med 344(14):1067–1078
Gerecht-Nir S, Itskovitz-Eldor J (2004) Cell therapy using human embryonic stem cells. Transpl Immunol 12(3–4):203–209
Gibson AJ, Karasinski J, Relvas J et al (1995) Dermal fibroblasts convert to a myogenic lineage in mdx mouse muscle. J Cell Sci 108(Pt 1):207–214
Gussoni E, Bennett RR, Muskiewicz KR et al (2002) Long-term persistence of donor nuclei in a Duchenne muscular dystrophy patient receiving bone marrow transplantation. J Clin Invest 110(6):807–814
He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ (2003) Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res 93(1):32–39
Heginbotham L, Lu Z, Abramson T, MacKinnon R (1994) Mutations in the K+ channel signature sequence. Biophys J 66(4):1061–1067
Herskowitz I (1987) Functional inactivation of genes by dominant negative mutations. Nature 329(6136):219–222
Hirano Y, Hiraoka M (1988) Barium-induced automatic activity in isolated ventricular myocytes from guinea-pig hearts. J Physiol 395:455–472
Hoffman LM, Carpenter MK (2005) Characterization and culture of human embryonic stem cells. Nat Biotechnol 23(6):699–708
Hoppe UC, Jansen E, Sudkamp M, Beuckelmann DJ (1998) Hyperpolarization-activated inward current in ventricular myocytes from normal, failing human hearts. Circulation 97(1):55–65
Hoppe UC, Johns DC, Marban E, O’Rourke B (1999) Manipulation of cellular excitability by cell fusion: effects of rapid introduction of transient outward K+ current on the guinea pig action potential. Circ Res 84(8):964–972
Imoto Y, Ehara T, Matsuura H (1987) Voltage- and time-dependent block of iK1 underlying Ba2+-induced ventricular automaticity. Am J Physiol 252(2 Pt 2):H325–H333
Irisawa H, Brown HF, Giles W (1993) Cardiac pacemaking in the sinoatrial node. Physiol Rev 73(1):197–227
Jalife J, Sicouri S, Delmar M, Michaels DC (1989) Electrical uncoupling and impulse propagation in isolated sheep Purkinje fibers. Am J Physiol 257(1 Pt 2):H179–H189
Kashiwakura Y, Cho HC, Azene E, Marban E (2005) Creation of a synthetic pacemaker channel. Circulation 114(16):1682–1686
Kehat I, Kenyagin-Karsenti D, Snir M et al (2001) Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest 108(3):407–414
Kehat I, Khimovich L, Caspi O et al (2004) Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol 22(10):1282–1289
Kohl P (2003) Heterogeneous cell coupling in the heart: an electrophysiological role for fibroblasts. Circ Res 93(5):381–383
Kubo Y, Baldwin TJ, Jan YN, Jan LY (1993) Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature 362(6416):127–133
Kusumoto FM, Goldschlager N (1996) Cardiac pacing. N Engl J Med 334(2):89–97
Lakatta EG, Maltsev VA, Bogdanov KY, Stern MD, Vinogradova TM (2003) Cyclic variation of intracellular calcium: a critical factor for cardiac pacemaker cell dominance. Circ Res 92(3):e45–e50
Lentz BR, Lee JK (1999) Poly(ethylene glycol) (PEG)-mediated fusion between pure lipid bilayers: a mechanism in common with viral fusion and secretory vesicle release? Mol Membr Biol 16(4):279–296
Loewenstein WR (1981) Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev 61(4):829–913
Losordo DW, Vale PR, Symes JF et al (1998) Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation 98(25):2800–2804
Mann MJ, Whittemore AD, Donaldson MC et al (1999) Ex-vivo gene therapy of human vascular bypass grafts with E2F decoy: the PREVENT single-centre, randomised, controlled trial. Lancet 354(9189):1493–1498
Martin CM, Meeson AP, Robertson SM et al (2004) Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol 265(1):262–275
Matsuura K, Nagai T, Nishigaki N et al (2004) Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem 279(12):11384–11391
Messina E, De Angelis L, Frati G et al (2004) Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res 95(9):911–921
Miake J, Marban E, Nuss HB (2002) Biological pacemaker created by gene transfer. Nature 419(6903):132–133
Miller AG, Aldrich RW (1996) Conversion of a delayed rectifier K+ channel to a voltage-gated inward rectifier K+ channel by three amino acid substitutions. Neuron 16(4):853–858
Mummery C, Ward-van Oostwaard D, Doevendans P et al (2003) Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation 107(21):2733–2740
Nuss HB, Johns DC, Kaab S et al (1996) Reversal of potassium channel deficiency in cells from failing hearts by adenoviral gene transfer: a prototype for gene therapy for disorders of cardiac excitability and contractility. Gene Ther 3(10):900–912
Nuss HB, Marban E, Johns DC (1999) Overexpression of a human potassium channel suppresses cardiac hyperexcitability in rabbit ventricular myocytes. J Clin Invest 103(6):889–896
Odorico JS, Kaufman DS, Thomson JA (2001) Multilineage differentiation from human embryonic stem cell lines. Stem Cells 19(3):193–204
Oh H, Bradfute SB, Gallardo TD et al (2003) Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA 100(21):12313–12318
Ohno T, Gordon D, San H et al (1994) Gene therapy for vascular smooth muscle cell proliferation after arterial injury. Science 265(5173):781–784
Pfister O, Mouquet F, Jain M et al (2005) CD31− but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res 97(1):52–61
Potapova I, Plotnikov A, Lu Z et al (2004) Human mesenchymal stem cells as a gene delivery system to create cardiac pacemakers. Circ Res 94(7):952–959
Ren D, Navarro B, Xu H, Yue L, Shi Q, Clapham DE (2001) A prokaryotic voltage-gated sodium channel. Science 294(5550):2372–2375
Richardson AW, Josephson ME (1999) Ablation of ventricular tachycardia in the setting of coronary artery disease. Curr Cardiol Rep 1(2):157–164
Robertson JA (2001) Human embryonic stem cell research: ethical and legal issues. Nat Rev Genet 2(1):74–78
Robinson RB, Siegelbaum SA (2003) Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol 65:453–480
Rodriguez-Contreras A, Nonner W, Yamoah EN (2002) Ca2+ transport properties and determinants of anomalous mole fraction effects of single voltage-gated Ca2+ channels in hair cells from bullfrog saccule. J Physiol 538(Pt 3):729–745
Rosengart TK, Lee LY, Patel SR et al (1999) Six-month assessment of a phase I trial of angiogenic gene therapy for the treatment of coronary artery disease using direct intramyocardial administration of an adenovirus vector expressing the VEGF121 cDNA. Ann Surg 230(4):466–470; discussion 70–72
Santoro B, Tibbs GR (1999) The HCN gene family: molecular basis of the hyperpolarization-activated pacemaker channels. Ann N Y Acad Sci 868:741–764
Shirahata S, Katakura Y, Teruya K (1998) Cell hybridization, hybridomas, and human hybridomas. Methods Cell Biol 57:111–145
Siebels J, Cappato R, Ruppel R, Schneider MA, Kuck KH (1993) Preliminary results of the Cardiac Arrest Study Hamburg (CASH). CASH Investigators. Am J Cardiol 72(16):109F–113F
Slesinger PA, Patil N, Liao YJ, Jan YN, Jan LY, Cox DR (1996) Functional effects of the mouse weaver mutation on G protein-gated inwardly rectifying K+ channels. Neuron 16(2):321–331
Smith R, Messina E, Abraham M, Cho HC, Giacomello A, Marban E (2005) Electrophysiology of human and porcine adult cardiac stem cells isolated from endomyocardial biopsies. Circulation 111(13):1720
Smith R, Barile L, Cho HC et al (2005) Unique phenotype of cardiospheres derived from human endomyocardial biopsies. Circulation 112(17):II-51
Sperelakis N (2002) An electric field mechanism for transmission of excitation between myocardial cells. Circ Res 91(11):985–987
Thom T, Haase N, Rosamond W et al (2006) Heart disease and stroke statistics-2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 113(6):e85–e151
Ulens C, Tytgat J (2001) Functional heteromerization of HCN1 and HCN2 pacemaker channels. J Biol Chem 276(9):6069–6072
van der Velden HM, Jongsma HJ (2002) Cardiac gap junctions and connexins: their role in atrial fibrillation and potential as therapeutic targets. Cardiovasc Res 54(2):270–279
Waldo AL, Camm AJ, deRuyter H et al (1996) Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD investigators. Survival with oral d-sotalol. Lancet 348(9019):7–12
Wang Z, Feng J, Shi H, Pond A, Nerbonne JM, Nattel S (1999) Potential molecular basis of different physiological properties of the transient outward K+ current in rabbit and human atrial myocytes. Circ Res 84(5):551–561
Weimann JM, Johansson CB, Trejo A, Blau HM (2003) Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat Cell Biol 5(11):959–966
Wier WG, Blatter LA (1991) Ca(2+)-oscillations and Ca(2+)-waves in mammalian cardiac and vascular smooth muscle cells. Cell Calcium 12(2–3):241–254
Wobus AM, Rohwedel J, Maltsev V, Hescheler J (1995) Development of cardiomyocytes expressing cardiac-specific genes, action potentials, and ionic channels during embryonic stem cell-derived cardiogenesis. Ann N Y Acad Sci 752:460–469
Xu C, Police S, Rao N, Carpenter MK (2002) Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res 91(6):501–508
Xue T, Marban E, Li RA (2002) Dominant-negative suppression of HCN1- and HCN2-encoded pacemaker currents by an engineered HCN1 construct: insights into structure-function relationships and multimerization. Circ Res 90(12):1267–1273
Xue T, Cho HC, Akar FG et al (2005) Functional integration of electrically active cardiac derivatives from genetically engineered human embryonic stem cells with quiescent recipient ventricular cardiomyocytes: insights into the development of cell-based pacemakers. Circulation 111(1):11–20
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marbán, E., Cho, H.C. Creation of a biological pacemaker by gene- or cell-based approaches. Med Bio Eng Comput 45, 133–144 (2007). https://doi.org/10.1007/s11517-007-0165-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-007-0165-2