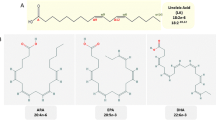
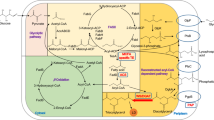
Abstract
More than 300 types of modified fatty acids (mFA) are produced in triacylglycerols (TAG) by various plant species, with many of these unusual structures rendering unique physical and chemical properties that are desirable for a variety of bio-based industrial uses. Attempts to produce these mFA in crop species have thus far failed to reach the desired levels of production and highlighted the need to better understand how fatty acids are synthesized and accumulated in seed oils. In this review we discuss how some of the progress made in recent years, such as the improved TAG synthesis model to include acyl editing and new enzymes such as PDCT, may be utilized to achieve the goal of effectively modifying plant oils for industrial uses. Co-expressing several key enzymes may circumvent the bottlenecks for the accumulation of mFA in TAG through efficient removal of mFA from phosphatidylcholine. Other approaches include the prevention of feedback inhibition of fatty acid synthesis and improving primary enzyme activity in host transgenic plants. In addition, genomic approaches are providing unprecedented power to discover more factors that may facilitate engineering mFA in oilseeds. Based on the results of the last 20 years, creating a high mFA accumulating plant will not be done by simply inserting one or two genes; it is necessary to stack genes encoding enzymes with favorable kinetic activity or specificity along with additional complementary transgenes in optimized plant backgrounds to produce industrial fatty acids at desirable levels. Finally, we discuss the potential of Camelina as an industrial oilseed platform.
Similar content being viewed by others
References
Andre C, Haslam R P, Shanklin J (2012). Feedback regulation of plastidic acetyl-CoA carboxylase by 18:1-acyl carrier protein in Brassica napus. Proc Natl Acad Sci USA,Online Available June 4, 2012
Bafor M, Smith M A, Jonsson L, Stobart K, Stymne S (1991). Ricinoleic acid biosynthesis and triacylglycerol assembly in microsomal preparations from developing castor-bean (Ricinus communis) endosperm. Biochem J, 280(Pt2): 507–514
Bao X, Katz S, Pollard M, Ohlrogge J (2002). Carbocyclic fatty acids in plants: biochemical and molecular genetic characterization of cyclopropane fatty acid synthesis of Sterculiafoetida. Proc Natl Acad Sci USA, 99(10): 7172–7177
Bates P D, Browse J (2011). The pathway of triacylglycerol synthesis through phosphatidylcholine in Arabidopsis produces a bottleneck for the accumulation of unusual fatty acids in transgenic seeds. Plant J, 68(3): 387–399
Bates P D, Durrett T P, Ohlrogge J B, Pollard M (2009). Analysis of acyl fluxes through multiple pathways of triacylglycerol synthesis in developing soybean embryos. Plant Physiol, 150(1): 55–72
Bates P D, Ohlrogge J B, Pollard M (2007). Incorporation of newly synthesized fatty acids into cytosolic glycerolipids in pea leaves occurs via acyl editing. J Biol Chem, 282(43): 31206–31216
Beilstein M A, Al-Shehbaz I A, Kellogg E A (2006). Brassicaceae phylogeny and trichome evolution. Am J Bot, 93(4): 607–619
Broadwater J A, Whittle E, Shanklin J (2002). Desaturation and hydroxylation. Residues 148 and 324 of Arabidopsis FAD2, in addition to substrate chain length, exert a major influence in partitioning of catalytic specificity. J Biol Chem, 277(18): 15613–15620
Broun P, Somerville C (1997). Accumulation of ricinoleic, lesquerolic, and densipolic acids in seeds of transgenic Arabidopsis plants that express a fatty acyl hydroxylase cDNA from castor bean. Plant Physiol, 113(3): 933–942
Brown A P, Kroon J T, Swarbreck D, Febrer M, Larson T R, Graham I A, Caccamo M, Slabas A R (2012). Tissue-specific whole transcriptome sequencing in castor, directed at understanding triacylglycerol lipid biosynthetic pathways. PLoS ONE, 7(2): e30100
Browse J, McConn M, James D Jr, Miquel M (1993). Mutants of Arabidopsis deficient in the synthesis of alpha-linolenate. Biochemical and genetic characterization of the endoplasmic reticulum linoleoyl desaturase. J Biol Chem, 268(22): 16345–16351
Browse J, Somerville C (1991). Glycerolipid synthesis — biochemistry and regulation. Annu Rev Plant Physiol, 42(1): 467–506
Burgal J, Shockey J, Lu C, Dyer J, Larson T, Graham I, Browse J (2008). Metabolic engineering of hydroxy fatty acid production in plants: RcDGAT2 drives dramatic increases in ricinoleate levels in seed oil. Plant Biotechnol J, 6(8): 819–831
Cahoon E B, Carlson T J, Ripp K G, Schweiger B J, Cook G A, Hall S E, Kinney A J (1999). Biosynthetic origin of conjugated double bonds: production of fatty acid components of high-value drying oils in transgenic soybean embryos. Proc Natl Acad Sci USA, 96(22): 12935–12940
Cahoon E B, Dietrich C R, Meyer K, Damude H G, Dyer J M, Kinney A J (2006). Conjugated fatty acids accumulate to high levels in phospholipids of metabolically engineered soybean and Arabidopsis seeds. Phytochemistry, 67(12): 1166–1176
Cahoon E B, Ripp K G, Hall S E, McGonigle B (2002). Transgenic production of epoxy fatty acids by expression of a cytochrome P450 enzyme from Euphorbia lagascae seed. Plant Physiol, 128(2): 615–624
Chan A P, Crabtree J, Zhao Q, Lorenzi H, Orvis J, Puiu D, Melake-Berhan A, Jones K M, Redman J, Chen G, Cahoon E B, Gedil M, Stanke M, Haas B J, Wortman J R, Fraser-Liggett C M, Ravel J, Rabinowicz P D (2010). Draft genome sequence of the oilseed species Ricinus communis. Nat Biotechnol, 28(9): 951–956
Collins-Silva J E, Lu C, Cahoon E B (2011). Camelina: a designer biotech oilseed crop. Inform, 22: 610–613
Dahlqvist A, Stahl U, Lenman M, Banas A, Lee M, Sandager L, Ronne H, Stymne S (2000). Phospholipid:diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc Natl Acad Sci USA, 97(12): 6487–6492
Dauk M, Lam P, Kunst L, Smith M (2007). A FAD2 homologue from Lesquerella lindheimeri has predominantly fatty acid hydroxylase activity. Plant Sci, 173(1): 43–49
Drexler H, Spiekermann P, Meyer A, Domergue F, Zank T, Sperling P, Abbadi A, Heinz E (2003). Metabolic engineering of fatty acids for breeding of new oilseed crops: strategies, problems and first results. J Plant Physiol, 160(7): 779–802
Durrett T P, Benning C, Ohlrogge J (2008). Plant triacylglycerols as feedstocks for the production of biofuels. Plant J, 54(4): 593–607
Durrett T P, McClosky D D, Tumaney A W, Elzinga D A, Ohlrogge J, Pollard M (2010). A distinct DGAT with sn-3 acetyltransferase activity that synthesizes unusual, reduced-viscosity oils in Euonymus and transgenic seeds. Proc Natl Acad Sci USA, 107(20): 9464–9469
Dyer J M, Mullen R T (2008). Engineering plant oils as high-value industrial feedstocks for biorefining: the need for underpinning cell biology research. Physiol Plant, 132(1): 11–22
Dyer J M, Stymne S, Green A G, Carlsson A S (2008). High-value oils from plants. Plant J, 54(4): 640–655
Eccleston V S, Cranmer A M, Voelker T A, Ohlrogge J B (1996). Medium-chain fatty acid biosynthesis and utilization in Brassica napus plants expressing lauroyl-acyl carrier protein thioesterase. Planta, 198(1): 46–53
Eccleston V S, Ohlrogge J B (1998). Expression of lauroyl-acyl carrier protein thioesterase in Brassica napus seeds induces pathways for both fatty acid oxidation and biosynthesis and implies a set point for triacylglycerol accumulation. Plant Cell, 10(4): 613–622
Gehringer A, Friedt W, Lühs W, Snowdon R J (2006). Genetic mapping of agronomic traits in false flax (Camelina sativa subsp. sativa). Genome, 49(12): 1555–1563
Goode J H, Dewey R E (1999). Characterization of aminoalcoholphosphotransferases from Arabidopsis thaliana and soybean. Plant Physiol Biochem, 37(6): 445–457
Gunstone F D (1998). Movements towards tailor-made fats. Prog Lipid Res, 37(5): 277–305
Hobbs D H, Lu C, Hills M J (1999). Cloning of a cDNA encoding diacylglycerol acyltransferase from Arabidopsis thaliana and its functional expression. FEBS Lett, 452(3): 145–149
Hu Z, Ren Z, Lu C (2012). The phosphatidylcholine diacylglycerol cholinephosphotransferase is required for efficient hydroxy Fatty Acid accumulation in transgenic Arabidopsis. Plant Physiol, 158(4): 1944–1954
Huber G W, Iborra S, Corma A (2006). Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chem Rev, 106(9): 4044–4098
Jaworski J, Cahoon E B (2003). Industrial oils from transgenic plants. Curr Opin Plant Biol, 6(2): 178–184
Kang J, Snapp A R, Lu C (2011). Identification of three genes encoding microsomal oleate desaturases (FAD2) from the oilseed crop Camelina sativa. Plant Physiol Biochem, 49(2): 223–229
Katavic V, Reed D W, Taylor D C, Giblin E M, Barton D L, Zou J, Mackenzie S L, Covello P S, Kunst L (1995). Alteration of seed fatty acid composition by an ethyl methanesulfonate-induced mutation in Arabidopsis thaliana affecting diacylglycerol acyltransferase activity. Plant Physiol, 108(1): 399–409
Kennedy E P (1961). Biosynthesis of complex lipids. Fed Proc, 20: 934–940
Kunst L, Taylor D C, Underhill E W (1992). Fatty acid elongation in developing seeds of Arabidopsis thaliana. Plant Physiol Biochem, 30: 425–434
Lee M, Lenman M, Banaś A, Bafor M, Singh S, Schweizer M, Nilsson R, Liljenberg C, Dahlqvist A, Gummeson P O, Sjödahl S, Green A, Stymne S (1998). Identification of non-heme diiron proteins that catalyze triple bond and epoxy group formation. Science, 280(5365): 915–918
Li R, Yu K, Hatanaka T, Hildebrand D F (2010). Vernonia DGATs increase accumulation of epoxy fatty acids in oil. Plant Biotechnol J, 8(2): 184–195
Li R, Yu K, Wu Y, Tateno M, Hatanaka T, Hildebrand D F (2012). Vernonia DGATs can complement the disrupted oil and protein metabolism in epoxygenase-expressing soybean seeds. Metab Eng, 14(1): 29–38
Lu C (2008). Camelina sativa: a potential oilseed crop for biofuels and genetically engineered products. In Information Systems for Biotechnology, 7–9
Lu C, Fulda M, Wallis J G, Browse J (2006). A high-throughput screen for genes from castor that boost hydroxy fatty acid accumulation in seed oils of transgenic Arabidopsis. Plant J, 45(5): 847–856
Lu C, Kang J (2008). Generation of transgenic plants of a potential oilseed crop Camelina sativa by Agrobacterium-mediated transformation. Plant Cell Rep, 27(2): 273–278
Lu C, Napier J A, Clemente T E, Cahoon E B (2011). New frontiers in oilseed biotechnology: meeting the global demand for vegetable oils for food, feed, biofuel, and industrial applications. Curr Opin Biotechnol, 22(2): 252–259
Lu C, Wallis J G, Browse J (2007). An analysis of expressed sequence tags of developing castor endosperm using a full-length cDNA library. BMC Plant Biol, 7(1): 42
Lu C, Xin Z, Ren Z, Miquel M, Browse J (2009). An enzyme regulating triacylglycerol composition is encoded by the ROD1 gene of Arabidopsis. Proc Natl Acad Sci USA, 106(44): 18837–18842
Moire L, Rezzonico E, Goepfert S, Poirier Y (2004). Impact of unusual fatty acid synthesis on futile cycling through beta-oxidation and on gene expression in transgenic plants. Plant Physiol, 134(1): 432–442
Moser B R (2010). Camelina (Camelina sativa L.) oil as a biofuels feedstock: golden opportunity or false hope? Lipid Technology, 22(12): 270–273
Napier J A, Graham I A (2010). Tailoring plant lipid composition: designer oilseeds come of age. Curr Opin Plant Biol, 13(3): 330–337
Nguyen H T, Mishra G, Whittle E, Pidkowich M S, Bevan S A, Merlo A O, Walsh T A, Shanklin J (2010). Metabolic engineering of seeds can achieve levels of omega-7 fatty acids comparable with the highest levels found in natural plant sources. Plant Physiol, 154(4): 1897–1904
Ohlrogge J, Browse J (1995). Lipid biosynthesis. Plant Cell, 7(7): 957–970
Okuley J, Lightner J, Feldmann K, Yadav N, Lark E, Browse J (1994). Arabidopsis FAD2 gene encodes the enzyme that is essential for polyunsaturated lipid synthesis. Plant Cell, 6(1): 147–158
Pilgeram A L, Sands D C, Boss D, Dale N, Wichman D, Lamb P, Lu C, Barrows R, Kirkpatrick M, Thompson B, Johnson D L (2007). Camelina sativa, a Montana omega-3 and fuel crop. In: Issues in new crops and new uses (Janick J, Whipkey A, eds.), Alexandria, VA: ASHS Press, 129–131
Pinzi S, Garcia I L, Lopez-Gimenez F J, de Castro M D L, Dorado G, Dorado M P (2009). The ideal vegetable oil-based biodiesel composition: a review of social, economical and technical implications. Energy Fuels, 23(5): 2325–2341
Riediger N D, Othman R A, Suh M, Moghadasian M H (2009). A systemic review of the roles of n-3 fatty acids in health and disease. J Am Diet Assoc, 109(4): 668–679
Roesler K, Shintani D, Savage L, Boddupalli S, Ohlrogge J (1997). Targeting of the Arabidopsis homomeric acetyl-coenzyme A carboxylase to plastids of rapeseeds. Plant Physiol, 113(1): 75–81
Roughan P G, Slack C R (1982). Cellular-organization of glycerolipid metabolism. Annu Rev Plant Physiol, 33(1): 97–132
Routaboul J M, Benning C, Bechtold N, Caboche M, Lepiniec L (1999). The TAG1 locus of Arabidopsis encodes for a diacylglycerol acyltransferase. Plant Physiol Biochem, 37(11): 831–840
Shintani D K, Ohlrogge J B (1995). Feedback inhibition of fatty acid synthesis in tobacco suspension cells. Plant J, 7(4): 577–587
Shockey J M, Fulda M S, Browse J A (2002). Arabidopsis contains nine long-chain acyl-coenzyme a synthetase genes that participate in fatty acid and glycerolipid metabolism. Plant Physiol, 129(4): 1710–1722
Shockey J M, Gidda S K, Chapital D C, Kuan J C, Dhanoa P K, Bland J M, Rothstein S J, Mullen R T, Dyer J M (2006). Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell, 18(9): 2294–2313
Singh S P, Zhou X R, Liu Q, Stymne S, Green A G (2005). Metabolic engineering of new fatty acids in plants. Curr Opin Plant Biol, 8(2): 197–203
Slack C R, Campbell L C, Browse J A, Roughan P G (1983). Some evidence for the reversibility of cholinephosphotransferase-catalyzed reaction in developing linseed cotyledons in vivo. Biochim Biophys Acta, 754: 10–20
Sperling P, Linscheid M, Stöcker S, Mühlbach H P, Heinz E (1993). In vivo desaturation of cis-delta 9-monounsaturated to cis-delta 9,12-diunsaturated alkenylether glycerolipids. J Biol Chem, 268(36): 26935–26940
Ståhl U, Stålberg K, Stymne S, Ronne H (2008). A family of eukaryotic lysophospholipid acyltransferases with broad specificity. FEBS Lett, 582(2): 305–309
Stålberg K, Ståhl U, Stymne S, Ohlrogge J (2009). Characterization of two Arabidopsis thaliana acyltransferases with preference for lysophosphatidylethanolamine. BMC Plant Biol, 9(1): 60
Steen E J, Kang Y, Bokinsky G, Hu Z, Schirmer A, McClure A, Del Cardayre S B, Keasling J D (2010). Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature, 463(7280): 559–562
Stobart K, Mancha M, Lenman M, Dahlqvist A, Stymne S (1997). Triacylglycerols are synthesised and utilized by transacylation reactions in microsomal preparations of developing safflower (Carthamus tinctorius L.) seeds. Planta, 203: 58–66
Stuitje A R, Verbree E C, van der Linden K H, Mietkiewska E M, Nap J P, Kneppers T J A (2003). Seed-expressed fluorescent proteins as versatile tools for easy (co)transformation and high-throughput functional genomics in Arabidopsis. Plant Biotechnol J, 1(4): 301–309
Suh M C, Schultz D J, Ohlrogge J B (2002). What limits production of unusual monoenoic fatty acids in transgenic plants? Planta, 215(4): 584–595
Thomæus S, Carlsson A S, Stymne S (2001). Distribution of fatty acids in polar and neutral lipids during seed development in Arabidopsis thaliana genetically engineered to produce acetylenic, epoxy and hydroxy fatty acids. Plant Sci, 161(5): 997–1003
Troncoso-Ponce M A, Kilaru A, Cao X, Durrett T P, Fan J, Jensen J K, Thrower N A, Pauly M, Wilkerson C, Ohlrogge J B (2011). Comparative deep transcriptional profiling of four developing oilseeds. Plant J, 68(6): 1014–1027
van de Loo F J, Broun P, Turner S, Somerville C (1995). An oleate 12-hydroxylase from Ricinus communis L. is a fatty acyl desaturase homolog. Proc Natl Acad Sci USA, 92(15): 6743–6747
van Erp H, Bates P D, Burgal J, Shockey J, Browse J (2011). Castor phospholipid:diacylglycerol acyltransferase facilitates efficient metabolism of hydroxy fatty acids in transgenic Arabidopsis. Plant Physiol, 155(2): 683–693
Visarada K B R S, Meena K, Aruna C, Srujana S, Saikishore N, Seetharama N (2009). Transgenic Breeding: Perspectives and Prospects. Crop Sci, 49(5): 1555–1563
Voelker T, Kinney A J (2001). Variations in the biosynthesis of seedstorage lipids. Annu Rev Plant Physiol Plant Mol Biol, 52(1): 335–361
Wallis J G, Browse J (2010). Lipid biochemists salute the genome. Plant J, 61(6): 1092–1106
Whittle E, Shanklin J (2001). Engineering delta 9–16:0-acyl carrier protein (ACP) desaturase specificity based on combinatorial saturation mutagenesis and logical redesign of the castor delta 9–18:0-ACP desaturase. J Biol Chem, 276(24): 21500–2150
Williams J P, Imperial V, Khan M U, Hodson J N (2000). The role of phosphatidylcholine in fatty acid exchange and desaturation in Brassica napus L. leaves. Biochem J, 349(Pt 1): 127–133
Xu J, Carlsson A S, Francis T, Zhang M, Hoffman T, Giblin ME, Taylor D C (2012). Triacylglycerol synthesis by PDAT1 in the absence of DGAT1 activity is dependent on re-acylation of LPC by LPCAT2. BMC Plant Biol, 12(1): 4
Yang P, Li X, Shipp M J, Shockey J M, Cahoon E B (2010). Mining the bitter melon (Momordica charantia L.) seed transcriptome by 454 analysis of non-normalized and normalized cDNA populations for conjugated fatty acid metabolism-related genes. BMC Plant Biol, 10(1): 250
Yu K, McCracken C T Jr, Li R, Hildebrand D F (2006). Diacylglycerol acyltransferases from Vernonia and Stokesia prefer substrates with vernolic acid. Lipids, 41(6): 557–566
Zhang M, Fan J, Taylor D C, Ohlrogge J B (2009). DGAT1 and PDAT1 acyltransferases have overlapping functions in Arabidopsis triacylglycerol biosynthesis and are essential for normal pollen and seed development. Plant Cell, 21(12): 3885–3901
Zheng Q, Li J Q, Kazachkov M, Liu K, Zou J (2012). Identification of Brassica napus lysophosphatidylcholine acyltransferase genes through yeast functional screening. Phytochemistry, 75: 21–31
Zou J, Wei Y, Jako C, Kumar A, Selvaraj G, Taylor D C (1999). The Arabidopsis thaliana TAG1 mutant has a mutation in a diacylglycerol acyltransferase gene. Plant J, 19(6): 645–653
Zubr J (1997). Oil-seed crop: Camelina sativa. Ind Crops Prod, 6(2): 113–119
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Snapp, A.R., Lu, C. Engineering industrial fatty acids in oilseeds. Front. Biol. 8, 323–332 (2013). https://doi.org/10.1007/s11515-012-1228-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11515-012-1228-9