Abstract
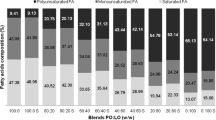
The effect of reaction time (0–90 min), catalyst concentration (0.2–0.5% CH3ONa powder), and temperature (60–90 °C) of chemical interesterification (CIE) was studied to determine the optimal conditions for maximal change of solid fat content (SFC) in minimal time in a beef tallow (BT)/canola oil (CaO) blend (80:20, w/w, herein after referred to as BT/CaO blend 80:20). The optimal conditions were obtained as: CH3ONa 0.4%, 60 °C, 30 min. BT/CaO blends (60:40, 65:35, 70:30, 75:25, 80:20, and 85:15) were each interesterified on a laboratory scale under afore-determined optimal conditions, and the corresponding 12 samples, before and after CIE, were characterized in terms of the SFC profile and compatibility. SFC profiling showed an increase in SFC (<5%) between 5 and 21.1 °C and a slight drop (<3%) in SFC between 40 and 45 °C for the interesterified blends. Compatibility analysis showed the presence of monotectic systems in original blends, proven through isothermal solid diagrams and isosolid diagrams. The incompatibility among the fats and oils was eliminated after reaction and the solution behavior shifted to a continuous solid solution. The interesterified 85:15, 80:20/75:25, 70:30, 65:35, and 60:40 BT/CaO blends displayed characteristics suited to application, respectively, for bakery shortenings, frying fats, all-purpose bakery shortenings, and bakery and roll-in margarines. The model shortenings produced from interesterified blends had more stable crystal morphology, crystal sizes, and double-chain (2L) stacking β′ polymorphs than blended shortenings under temperature fluctuation storage. Sensory analysis also showed that the former had less graininess and better spreadability than the latter during storage.









Similar content being viewed by others
References
Q.Z. Jin, H.Y. Gao, L. Shan, Y.F. Liu, X.G. Wang, Study on grainy crystals in edible beef tallow shortening. Food Res. Int. 40, 909–914 (2007)
F. Luddy, J. Hampson, S. Herb, H. Rothbart, Development of edible tallow fractions for specialty fat uses. J. Am. Oil Chem. Soc. 50, 240–244 (1973)
T.J. Weiss, G.A. Jacobson, L.H. Wiedermann, Reaction mechanics of sodium methoxide treatment of lard. J. Am. Oil Chem. Soc. 38, 396–399 (1961)
H. Konishi, W.E. Neff, T.L. Mounts, Chemical interesterification with regioselectivity for edible oils. J. Am. Oil Chem. Soc. 70, 411–415 (1993)
D. Rousseau, K. Forestière, A.R. Hill, A.G. Marangoni, Restructing butterfat through blending and chemical interesterification. 1. Melting behavior and triacylglycerol modifications. J. Am. Oil Chem. Soc. 73, 963–972 (1996)
R. Grimaldi, L.A.G. Gonçalves, M.Y. Ando, Otimização da reação de interesterificação química de óleo de palma. Quim. Nova 28, 633–636 (2005)
D. Firestone, Official Methods and Recommended Practices of the American Oil Chemists’ Society, 5th edn. (American Oil Chemists’ Society, Champaign, 2004)
S. Braipson-Danthine, C. Deroanne, Determination of solid fat content (SFC) of binary fat blends and use of these data to predict SFC of selected ternary fat blends containing low-erucic rapeseed oil. J. Am. Oil Chem. Soc. 83, 571–581 (2006)
S. Braipson-Danthine, C. Deroanne, Influence of SFC, microstructure and polymorphism on texture (hardness) of binary blends of fats involved in the preparation of industrial shortenings. Food Res. Int. 37, 941–948 (2004)
L.B. Hare, Mixture designs applied to food formulation. Food Technol. 28, 50–62 (1974)
F.D. Gunstone, Fatty Acid and Lipid Chemistry (Blackie Academic & Professional, London, 1996), pp. 205–222
C.E. Høy, X.B. Xu, Structured Triacylglycerols, in Structured and Modified Lipids, ed. by F.D. Gunstone (CRC, New York, 2001), pp. 209–240
F.A.S.M. Soares, R.C. Silva, K.C.G. Silva, M.B. Lourenço, D.F. Soares, L.A. Gioielli, Effects of chemical interesterification on physicochemical properties of blends of palm stearin and palm olein. Food Res. Int. 42, 1287–1294 (2009)
R.C. Silva, L.N. Cotting, T.P. Poltronieri, V.M. Balcão, D.B. Almeida, L.A.G. Goncalves, R. Grimaldi, L.A. Gioielli, The effects of enzymatic interesterification on the physical–chemical properties of blends of lard and soybean oil. LWT Food Sci. Tech. 42, 1275–1282 (2009)
R. Grimaldi, L.A.G. Goncalves, L.A. Gioielli, I.S. Simões, Interactions in interesterified palm and palm kernel oils mixtures. I—Solid fat content and consistency. Grasas Aceites 52, 349–354 (2001)
R.E. Timms, Phase behaviour of fats and their mixtures. Prog. Lipid Res. 23, 1–38 (1984)
A.P.B. Ribeiro, R. Grimaldi, L.A. Gioielli, L.A.G. Gonçalves, Zero trans fats from soybean oil and fully hydrogenated soybean oil: Physico-chemical properties and food applications. Food Res. Int. 42, 401–410 (2009)
D. Rousseau, A.G. Marangoni, Chemical Interesterification of Food Lipids: Theory and Practice, in Food Lipids: Chemistry, Nutrition, and Biotechnology, ed. by C.C. Akoh, D.B. Min (CRC, New York, 2002), pp. 301–335
K.L. Humphrey, S.S. Narine, Lipid Phase Behavior, in Fat Crystal Networks, ed. by A.G. Marangoni (Marcel Dekker, New York, 2005), pp. 83–115
Y.F. Liu, Z. Meng, L. Shan, Q.Z. Jin, X.G. Wang, Preparation of specialty fats from beef tallow and canola oil by chemical interesterification: Physico-chemical properties and bread applications of the products. Eur. Food Res. Technol. 230, 457–466 (2010)
R.C. Silva, D.F. Soares, M.B. Lourenço, F.A.S.M. Soares, K.G. Silva, M.I.A. Gonçalves, L.A. Gioielli, Structured lipids obtained by chemical interesterification of olive oil and palm stearin. LWT Food Sci. Tech. 43, 752–758 (2010)
I. Karabulut, S. Turan, G. Ergin, Effects of chemical interesterification on solid fat content and slip melting point of fat/oil blends. Eur. Food Res. Technol. 218, 224–229 (2004)
M. Aguedo, E. Hanon, S. Danthine, M. Paquot, G. Lognay, A. Thomas, M. Vandenbol, P. Thonart, J.P. Wathelet, C. Blecker, Enrichment of anhydrous milk fat in polyunsaturated fatty acid residues from linseed and rapeseed oils through enzymatic interesterification. J. Agric. Food Chem. 56, 1757–1765 (2008)
B.S. Ghotra, S.D. Dyal, S.S. Narine, Lipid shortenings: A review. Food Res. Int. 35, 1015–1048 (2002)
L. Ahmadi, A.G. Marangoni, Functionality and physical properties of interesterified high oleic shortening structured with stearic acid. Food Chem. 117, 668–673 (2009)
R.D. O’Brien, Fats and Oils—Formulating and Processing for Applications, 3rd edn. (CRC, Boca Raton, 2009), pp. 361–396
A. Watanabe, I. Tashima, N. Matsuzakj, J. Kurashige, K. Sato, On the formation of granular crystals in fat blends containing palm oil. J. Am. Oil Chem. Soc. 69, 1077–1080 (1992)
S. Miura, H. Konishi, Crystallization behavior of 1,3-dipalmitoyl-2-oleoyl-glycerol and 1-palmitoyl-2,3-dioleoylglycerol. Eur. J. Lipid Sci. Technol. 103, 804–809 (2001)
D. Rousseau, A.G. Marangoni, K.R. Jeffrey, The influence of chemical interesterification on the physicochemical properties of complex fat systems. 2. Morphology and polymorphism. J. Am. Oil Chem. Soc. 75, 1833–1839 (1998)
A.P.B. Ribeiro, R.C. Basso, R. Grimaldi, L.A. Gioielli, A.O. Santos, L.P. Cardoso, L.A.G. Gonçalves, Influence of chemical interesterification on thermal behavior, microstructure, polymorphism and crystallization properties of canola oil and fully hydrogenated cottonseed oil blends. Food Res. Int. 42, 1153–1162 (2009)
J.H. Lee, C.C. Akoh, D.S. Himmelsbach, K.-T. Lee, Preparation of interesterified plastic fats from fats and oils free of trans fatty acid. J. Agric. Food Chem. 56, 4039–4046 (2008)
S.D. Campbell, H.D. Goff, D. Rousseau, Comparison of crystallization properties of a palm stearin/canola oil blend and lard in bulk and emulsified form. Food Res. Int. 35, 935–944 (2002)
A.P.B. Ribeiro, R.C. Basso, R. Grimaldi, L.A. Gioielli, L.A.G. Gonçalves, Instrumental methods for the evaluation of interesterified fats. Food Anal. Methods 2, 282–302 (2009)
C. Lopez, P. Lesieur, G. Keller, M. Ollivon, Thermal behaviour of milk fat: 1. Unstable species of cream. J. Colloid Interface Sci. 229, 62–71 (2000)
Acknowledgments
This work was supported by the National High Technology Research and Development Program (863 Program) of China (contract no. 2010AA101506) and PhD research fund of Jiangnan University. The authors are grateful to Kerry Specialty Fats (Shanghai) Ltd. for providing materials for the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meng, Z., Liu, Y., Shan, L. et al. Specialty Fats from Beef Tallow and Canola Oil: Establishment of Reaction Conditions, Characterization of Products, and Evaluation of Crystal Stability. Food Biophysics 6, 115–126 (2011). https://doi.org/10.1007/s11483-010-9186-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-010-9186-8