Abstract
Methamphetamine (METH) is a frequent drug of abuse in U.S. populations and commonly associated with psychosis. This may be a factor in frequent criminal justice referrals and lengthy treatment required by METH users. Persecutory delusions and auditory hallucinations are the most consistent symptoms of METH-associated psychosis (MAP). MAP has largely been studied in Asian populations and risk factors have varied across studies. Duration, frequency and amount of use as well as sexual abuse, family history, other substance use, and co-occurring personality and mood disorders are risk factors for MAP. MAP may be unique with its long duration of psychosis and recurrence without relapse to METH. Seven candidate genes have been identified that may be associated with MAP. Six of these genes are also associated with susceptibility, symptoms, or treatment of schizophrenia and most are linked to glutamatergic neurotransmission. Animal studies of pre-pulse inhibition, attenuation of social interaction, and stereotypy and alterations in locomotion are used to study MAP in rodents. Employing various models, rodent studies have identified neuroanatomical and neurochemical changes associated with METH use. Throughout this review, we identify key gaps in our understanding of MAP and suggest potential directions for future research.
Similar content being viewed by others
Introduction
Methamphetamine (METH), a member of the amphetamine family of drugs of abuse, is easily manufactured and readily available in the USA. In 2008, approximately 13,000,000 or 5% of persons age 12 and older in the USA reported having used METH in their lifetime. While this decrease from 6.5% in 2002 (NSDUH 2009) is significant, subgroups remain particularly vulnerable to METH use disorders (MUD). For example, rural persons are more likely to use the drug than persons in either small or large metropolitan areas; and a recent analysis of the National Survey on Drug Use and Health noted that METH use increased as the setting became more rural (Lambert et al. 2008). Of concern, young adults in rural areas were nearly twice as likely as their urban counterparts (2.9 versus 1.5%, p < 0.001) to have used METH. Additionally, non-Hispanic whites entering Substance Use Disorders (SUD) treatment are most likely to identify opiates and marijuana as their illicit drugs of choice; whereas, Hispanic and Asian Americans are more likely to identify METH/amphetamine as their primary drug of choice (SAMHSA 2009). Lastly, gay and bisexual men use METH at much higher rates than other populations (Shoptaw et al. 2003). Significantly, U.S. youth had the second highest rate of amphetamine use worldwide in 2007 (UNODC 2009). Additionally, persons with METH use disorders entering SUD treatment are more likely to be referred by the criminal justice system than all other SUD admissions combined (59 versus 38%) and over twice as likely to receive long-term residential treatment than all other admissions (17 versus 8%) (SAMHSA 2009). It is unknown why METH use results in greater criminal justice-related admissions or longer treatment stays, but drug-associated psychotic symptoms may have a role in the drug’s unique behavioral effects. While amphetamines such as METH can precipitate and exacerbate psychotic symptoms in persons with schizophrenia (Batki and Harris 2004), it has long been recognized that such drug use can produce psychotic symptoms even in persons with no history of a primary psychotic disorder (Chen et al. 2003; McKetin et al. 2006). With these issues in mind, the goals of this review are to describe the risk factors, frequency, symptoms, and clinical implications of METH-associated psychosis (MAP), discuss candidate genes with significant associations, and review preclinical animal research that aims to simulate an understanding of MAP. Within each of these areas, we will discuss gaps in our current knowledge and potential directions for future research.
Clinical features
Natural history and epidemiology of MAP
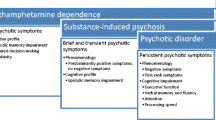
METH, cocaine, cannabis, alcohol, hallucinogens, sedatives, and heroin have all been implicated in substance-induced psychosis (Caton et al. 2005; van Os et al. 2002; Fergusson et al. 2003; Arseneault et al. 2004; Manschreck et al. 1988). Historical features such as psychotic symptoms presenting before onset of substantial substance use or psychotic symptoms only occurring in the context of substance use are useful in establishing a diagnosis. However, in many clinical settings the history is less clear and discriminating between psychoses that are secondary to substance use such as METH and those that are primary psychotic illnesses in a substance-using individual can be diagnostically challenging. In a study of 400 subjects recruited from five psychiatric emergency departments with at least one psychotic symptom and some substance use in the previous 30 days, 44% of subjects were diagnosed with a substance-induced psychosis while 56% were diagnosed with primary psychosis. The Psychiatric Research Interview for Substance and Mental Disorders (PRISM), which employs the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria for psychotic disorders, was utilized to discriminate between substance-induced and primary psychosis. Of note, a diagnosis of primary psychosis was made if there was “no evidence of heavy substance use or withdrawal, when psychotic symptoms persisted for at least 4 weeks in the absence of heavy substance use, or when psychotic symptoms preceded onset of heavy use.” Parental substance abuse, drug dependence (rather than abuse or use), and visual hallucinations were predictive of a substance-induced psychosis. Additionally, persons with substance-induced psychosis had lower Positive and Negative Syndrome Scale (PANSS) scores, greater awareness of psychotic symptoms and were more likely to have suicidal ideation (Caton et al. 2005). Similarly, in a small study (N = 19) of stimulant (cocaine or amphetamine) abusers seen in an emergency setting with psychotic symptoms determined by the Structured Clinical Interview for DSM-IV, positive rather than negative psychotic symptoms predominated with all participants reporting persecutory delusions, and most having delusions of reference and some form of hallucinations (Harris and Batki 2000). While these studies are useful in characterizing substance use-related psychosis, as will be discussed later in relation to METH, utilizing duration of psychotic symptoms as a key determinant in distinguishing primary psychosis from substance-induced psychosis may not be valid in all clinical settings.
MAP has been most consistently described in Japanese populations typically associated with longstanding METH use and characterized as resembling paranoid schizophrenia. Attempting to distinguish psychiatric disease symptoms that are appropriate to the situations within which METH users typically find themselves (purchasing, using, distributing and/or manufacturing the drug or engaging in other illegal activities), those symptoms which are unmasked by METH use but are related to an underlying psychotic illness (e.g., schizophrenia), and those symptoms which are secondary to the drug itself can be challenging. Nevertheless, a subset of METH users develops frank psychotic symptoms across a range from mild paranoia to hallucinations or unusual thought content.
In previous studies of METH users, Japanese, Taiwanese, Australian, and U.S. investigators have identified factors associated with MAP. In early Japanese studies (published 1955–1992), longer duration and more frequent METH use were associated with MAP (Ujike and Sato 2004). Of note, during this time period, Japanese METH users typically used METH exclusively in contrast to the poly-drug use seen in typical U.S. METH users (Ujike and Sato 2004). In 2003, Chen characterized three groups of Taiwanese METH users who were either hospitalized or in a detention center: those with no history of any psychotic symptoms, those with brief psychosis (less than 1 month after last METH use), and those with prolonged psychosis (psychosis which persisted more than 1 month after last METH use). In this study, those with MAP had earlier first METH use and used larger amounts of METH than those who had no history of psychotic symptoms. There was no difference in duration of METH use between the psychosis and non-psychosis group. There were, however, significant pre-morbid schizoid/schizotypal personality trait scores in those with METH-associated psychosis, and there was a significant linear correlation between these trait scores and presence and duration of psychosis. Additionally, the psychosis group had greater alcohol dependence, anti-social personality disorder (ASPD), and major depression (MDD) (Chen et al. 2003). In the same population, the first-degree relatives of those with psychosis were more likely to have schizophrenia than those METH users who had never had psychosis (OR = 5.4, 95% CI: 2.0–14.7, p < 0.001). Further, the risk for schizophrenia in the first degree relatives of those with prolonged psychosis was greater than those METH users with brief psychosis (OR = 2.8, 95% CI: 1.0–8.0, p = 0.042). Chen noted that the greater the “familial loading for schizophrenia, the more likely a METH user is to develop psychosis and the longer that psychosis is likely to last” (Chen et al. 2005).
In an Australian study of community METH users, McKetin found that MAP typically occurred in the context of METH abuse or dependence rather than “recreational” METH use. In METH users with no prior history of psychosis, the prevalence of psychosis among dependent users was 27% as compared to 8% in non-dependent users. However, daily METH use, injection use, and socio-demographic factors were not associated with METH psychosis (McKetin et al. 2006). In Japanese and Australian studies, method of METH administration (injection versus smoking) did not affect frequency of psychosis (Matsumoto et al. 2002; McKetin et al. 2008). In a study of adults with METH dependence in SUD treatment in the U.S., sexual abuse, greater frequency of recent METH use, and METH use combined with other drug use were associated with psychotic symptoms (Christian et al. 2007). In another U.S. study (N = 39) of persons with METH dependence who reported either frequent or infrequent psychosis while intoxicated on METH, there was no difference between the two groups in measures of intelligence, education, age of first METH use, or duration of METH use. However, those with frequent psychosis reported greater childhood attention deficit disorder (ADHD) and family history of psychiatric illness. Of the participants who had a positive family history for psychiatric illness, 67% had frequent psychotic episodes (Salo et al. 2008). In a small U.S. study (N = 19) of individuals being seen in an emergency setting, higher quantitative plasma METH and amphetamine levels were associated with more severe psychotic symptoms, but not to reported amounts of METH ingestion (Batki and Harris 2004). These studies did not identify factors which were consistently associated with MAP. However, the variability in the populations studied (e.g. community v. hospitalized), methamphetamine severity (use v. dependence) or use histories (single drug v. poly-drug) may have contributed to this inconsistency.
While METH psychosis has been discussed in the medical literature since the 1950s, previous studies examining the frequency of MAP have employed varying definitions of the disorder. Additionally, not all of the studies used standardized instruments to measure psychotic symptoms or described the time period (e.g., lifetime, current) within which psychosis was examined. Further, many of these studies (written in Japanese) are not accessible to all investigators. However, in a summary article, Sato noted that in previous Japanese studies more than 76% of METH users had a “paranoid psychotic state with hallucinations” (Sato 1992). In a comparison of non-treatment seeking cocaine- and METH-dependent men and women, METH-dependent individuals were more likely to report psychotic symptoms than cocaine-dependent men and women (Mahoney et al. 2008). While noting the above limitations in definition and diagnosis, as well as the variety of populations studied, in recent studies between 26 and 46% of persons with METH dependence have MAP (see Table 1).
Signs and symptoms of MAP
While it has been difficult to identify factors consistently associated with MAP or to precisely determine the frequency of MAP, the characterization of MAP symptoms across populations has been quite consistent. Multiple studies in Japanese (Akiyama 2006), Taiwanese (Chen et al. 2003), Australian (McKetin et al. 2006), and Thai (Srisurapanont et al. 2003) populations have described with remarkable consistency a high frequency of persecutory delusions and auditory hallucinations in persons with METH-related psychosis. Other frequently reported symptoms were delusions of reference, visual hallucinations, and thought broadcasting (Chen et al. 2003; Srisurapanont et al. 2003; Akiyama 2006). Ujike and Sato contend that there is a progression of MAP with an initial phase of psychotomimetic effects such as stimulation and heightened concentration, followed by “prepsychosis” with delusions, which may then progress to frank psychosis with hallucinations and delusions of reference (Ujike and Sato 2004). In a study of 149 METH users, the average latency from first use of METH to onset of psychosis was 5.2 years (Ujike and Sato 2004), while a study comparing METH smokers and METH injectors found a latency of 1.7 years in smokers and 4.4 years in injectors (Matsumoto et al. 2002). Severity of psychotic symptoms has been associated with frequency of METH use, METH injection (Zweben et al. 2004), and severity of METH craving (Nakama et al. 2008).
Most episodes of substance-induced and MAP are of brief duration and resolve as substance levels diminish. However, Japanese investigators have reported that while MAP may be transient with recovery within 1 month of last METH use, METH psychosis may also be prolonged, persisting for longer than 6 months (Ujike and Sato 2004), and may persist despite drug abstinence (Akiyama 2006). Western investigators have been more hesitant to attribute persistent psychotic symptoms solely to substance use and this is reflected in the diagnostic lexicon of the Diagnostic and Statistical Manual-IV which defines substance-induced psychosis as persisting for 1 month or less after last substance use (APA 2000). In contrast, in a Japanese study of 104 hospitalized METH users with no prior history of non-METH-related psychosis, 52% of participants’ psychotic symptoms abated within 1 week; whereas, in 26% of participants symptoms persisted for more than 1 month and in 16% of participants symptoms persisted for more than 3 months. Symptoms of psychosis were similar between the transient (duration < 1 week) and persistent (duration > 3 months) groups except that persons with persistent psychosis reported greater non-auditory and non-visual hallucinations (Iwanami et al. 1994). In a Taiwanese study of 174 METH users with psychotic symptoms, 17% had psychosis despite being abstinent for more than 1 month (Chen et al. 2003). In both studies, study participants were abstinent and had no history of schizophrenia or prior psychoses. It is unclear if long duration of MAP reflects the drug’s ability to cause a chronic psychotic disorder or if METH is unmasking a psychotic disorder in persons with an underlying psychotic diathesis. As previously noted, chronic psychotic symptoms associated with METH are more likely to occur in those with a family history of schizophrenia or in persons with a premorbid schizoid/schizotypal personality (Chen et al. 2003, 2005). Additionally, a previous neurological disorder (head injury, ADHD, prematurity, learning disability) may increase the risk of treatment-resistant psychosis in METH users (Fujii 2002).
Individuals with a brief METH-related psychosis may relapse to MAP with resumption of METH use or with a stressor such as severe insomnia or heavy alcohol use without METH consumption (Sato 1992; Yui et al. 2000; Ujike and Sato 2004). When MAP recurred with re-exposure to METH the symptoms were nearly identical to those in previous psychotic episodes (Sato 1992). If relapse to psychosis follows METH use, it typically occurs promptly with 60% of METH users relapsing in less than 1 week and 80% relapsing within 1 month. Spontaneous relapse usually occurred in persons with greater than 2 years of METH abuse and vulnerability to relapse provoked by re-use of METH persists for years (Ujike and Sato 2004).
Treatment of MAP
In multiple prior studies, the presence of both psychiatric illness and substance use worsened SUD treatment outcome (Rounsaville et al. 1991; Moos et al. 1994; Weisner et al. 2000). In a study of 526 METH dependent adults, individuals with any history of psychotic disorder at the 3-year follow-up were more than twice as likely to have been hospitalized in the previous 12 months, had more total hospitalizations, had greater severity of their psychiatric symptoms, and were more likely to have attempted suicide than participants with no history of psychosis. However, the two groups did not differ in their duration of SUD treatment attendance, treatment retention, or METH use. This study, however, did not distinguish between primary and substance-induced psychosis and those who required hospitalization were not eligible for study enrollment (Glasner-Edwards et al. 2008). Also of note, in a study of paranoia in community and in-treatment METH users, 37% of those persons with paranoia obtained a weapon, 11% used a weapon, and 15% attacked another person (Leamon et al. 2010). Only one randomized controlled trial evaluated anti-psychotics in persons with amphetamine-related psychosis. In this 4-week study, there was no significant difference in clinical efficacy between olanzapine and haloperidol (Leelahanaj et al. 2005).
Needs in clinical research
Studies of risk factors for MAP have largely been done in Japanese, Taiwanese, and Australian populations. It is unknown if findings from these large studies are generalizable to other populations such as those in the U.S. given the likely differences in use patterns, socio-environmental factors, and genetics. A single large study of U.S. METH users examined abuse and METH use characteristics and their association with psychosis. However, there are no studies of factors such as perceived stress or cultural stress which have been identified in other populations as being factors in the development of psychoses. Similarly, there are no studies of any kind assessing protective factors for MAP, which may mitigate against risk factors. Of note, none of the above studies examined sleep deprivation as a factor in the genesis of MAP, a factor that is well-described as a stressor which can result in psychosis. Additionally, there are no studies examining what role preexisting conditions, socio-environmental stressors or protective factors play in the severity, persistence or relapse of MAP. Lastly, little is known about the prevention of persistent and recurrent MAP.
Susceptibility genes
MAP is likely a complex genetic disease in which environmental factors interact with multiple polymorphic genes to influence susceptibility. Several studies have reported associations between MAP and genetic variation, including single nucleotide polymorphisms (SNPs), variable number tandem repeats (VNTRs), and insertion/deletions (I/D) (see Table 2). The candidate genes listed in Table 2 were selected based on a wide range of evidence, such as biological function, differential expression in disease, involvement in schizophrenia, which is considered pharmacologically similar to MAP, and findings from animal models. In these studies, allele frequencies were compared in unaffected and affected individuals. Unaffected individuals were generally healthy individuals without a history of psychosis-related disorders. Affected individuals were diagnosed using the ICD-10 or the DSM-IV criteria and were primarily individuals with METH dependence and psychosis (20% of studies reported individuals with METH abuse and psychosis). Of note, several studies consisted of heterogeneous populations that included individuals with and without psychosis. (These populations are annotated as such in Table 2.) Another phenotype often considered in these studies was the clinical course of MAP. The prognosis of MAP varied among individuals, some of whom showed continued psychotic symptoms, even after METH was discontinued. Accordingly, the individuals were categorized based on the duration of the psychotic state after METH discontinuation. Transient psychosis was defined as symptoms that improved within 1 month of METH discontinuation and the start of treatment with neuroleptics; whereas, the prolonged type was defined as psychotic symptoms continuing for more than 1 month after METH discontinuation and the treatment of neuroleptics. The hypothesis-driven studies investigating putative candidate genes and their association with psychosis among METH-dependent individuals are summarized in Table 2. Recognizing the high probability of false-positive associations due to multiple comparisons, we chose to focus on candidate genes with significant associations, i.e., p value < 0.001. There were seven genes in this category: D-amino acid oxidase activator, Dystrobrevin-Binding Protein 1, Frizzled 3, Metabotropic Glutamate Receptor 2, 5-Hydroxytryptamine (serotonin) Receptor 1A, Prokineticin Receptor 2, and Glycine Transporter 1.
D-amino acid oxidase activator (DAOA)
DAOA is the gene encoding the d-amino acid oxidase activator. DAOA is expressed in multiple tissues including the amygdala, caudate nucleus, spinal cord and testes (Chumakov et al. 2002). Although the functional mechanisms of DAOA are not fully understood, DAOA activates D-amino acid oxidase, which oxidizes d-serine, an endogenous ligand for the glycine site of the N-Methyl-D-aspartate (NMDA) type glutamate receptor (Chumakov et al. 2002). D-serine levels are low in schizophrenic patients and administration of D-serine has been shown to reduce some of the symptoms of this disease (Kantrowitz et al. 2010). This provides a potential link between DAOA and the glutamate hypo-function hypothesis of schizophrenia, which integrates environmental influences and causative genes, is based on clinical and neuropathological evidence of the hypo-function of glutamatergic signaling via NMDA receptors. The DAOA gene is located on chromosome 13q and has been found to be a susceptibility locus for schizophrenia (Badner and Gershon 2002), and many studies have found an association between genetic variants in DAOA and schizophrenia (Chumakov et al. 2002; Schumacher et al. 2004; Yue et al. 2007). In a case-controlled study, Kotaka et al. (2009) demonstrated an association between a DAOA polymorphism (rs778293; p = 0.0002) and psychosis among METH-dependent subjects. This polymorphism was previously shown to be associated with schizophrenia; however, its functionality is unknown.
Dystrobrevin-binding protein 1 (DTNBP1)
DTNBP1 encodes dystrobrevin-binding protein 1 also referred to as dysbindin. This ubiquitously expressed, coiled-coil-containing protein is a subunit of the biogenesis of lysosome-related organelles complex 1 (BLOC-1), which regulates trafficking to lysosome-related organelles (Li et al. 2003). In muscle and non-muscle cells, DTNBP1 binds to α- and β-dystrobrevins, components of the dystrophin-associated protein complex (DPC) (Benson et al. 2001). In muscle, the DPC is required for the maintenance of muscle integrity and normal muscle function. In the brain, dysbindin is most prevalent in axons, particularly those with large synaptic terminals such as the mossy fiber synaptic terminals in the cerebellum and hippocampus (Benson et al. 2001). In a case controlled study, Kishimoto et al. (2008a) demonstrated an association of a polymorphism in the DTNBP1 gene and psychosis among METH-dependent individuals (rs3213207; p = 0.000025). Furthermore, two haplotypes at these loci were also significantly associated with MAP (p values < 0.0015). Significant associations between schizophrenia and DTNBP1 also have been reported (Edwards et al. 2008; Straub et al. 2002). Consistent with this, reduced levels of dysbindin expression have been associated with the pathogenesis of schizophrenia (Talbot et al. 2004; Weickert et al. 2008) and thought to be related to glutamatergic neurotransmission. In schizophrenic patients, dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampus which is inversely correlated with increased vesicular glutamate transporter (Talbot et al. 2004). As evidence is mounting that glutamate hypo-function may be related to the etiology of schizophrenia (Konradi and Heckers 2003), it is postulated that DTNBP1 variants may contribute to MAP through pathways involving glutamatergic neurotransmission. Despite the many genetic studies of this gene, the link between functionality of these polymorphisms and dysbindin expression has not been investigated.
Frizzled 3 (FZD3)
Frizzled proteins are cell surface receptors for secreted Wnt proteins (Wang et al. 2006a). Both frizzled and Wnt proteins are thought to be important in central nervous system (CNS) development. FZD3, the human Frizzled-3 gene, is widely expressed in the developing nervous system and is involved in axonal growth and guidance (Wang et al. 2006b). Wnt signaling plays a role in axonal remodeling and synaptic differentiation in the cerebellum (Cadet and Krasnova 2009; Lucas and Salinas 1997) and abnormal Wnt signaling has been linked to schizophrenia (Cotter et al. 1998). FZD3 maps to chromosome 8p21 and consists of eight exons. The 8p22-21 region has been identified as a susceptibility locus for schizophrenia in several studies (Blouin et al. 1998; Gurling et al. 2001; Pulver et al. 1995). However, genetic studies with the Frizzled-3 gene and schizophrenia have been contradictory, with both positive and negative associations among Han Chinese (Yang et al. 2003), Japanese (Zhang et al. 2004), and Korean (Jeong et al. 2006) populations. Interestingly, the association between Frizzled-3 and MAP has only been observed with haplotypes (p < 0.00002) and not at the individual loci, which may indicate a required synergism between the polymorphisms for a phenotypic effect (Kishimoto et al. 2008b).
Metabotropic glutamate receptor 2 (GRM2)
GRM2 is another gene involved in glutamatergic neurotransmission and found to be significantly associated with MAP (Tsunoka et al. 2010). GRM2 is located on chromosome 3p in a region linked to schizophrenia in several studies (Badner and Gershon 2002; Pulver et al. 1995). GRM2 encodes the group II metabotropic glutamate receptor 2 (mGlu2). mGlu2 is a G-protein coupled receptor involved in inhibition of adenylate cyclase and cAMP formation (Cartmell et al. 1999) and the major presynaptic group II autoreceptor activated by synaptically released glutamate (Kew et al. 2002). Although another group II metabotropic glutamate receptor, glutamate receptor 3 (mGlu3), has been implicated in etiological, pathophysiological and pharmacotherapeutic aspects of schizophrenia (Harrison et al. 2008; Lyon et al. 2008; Sartorius et al. 2008), no statistically significant association with GRM2 and schizophrenia was found in the Japanese population (Joo et al. 2001).
5-Hydroxytryptamine (Serotonin) receptor 1A (5-HT1A)
5-HT1A, the gene encoding the 5-HT receptor 1A, is a G protein-coupled receptor which is widely expressed in the brain, including in the hypothalamus, hippocampus, and cortex. Through serotonin (5-HT) binding, this receptor mediates inhibitory neurotransmission. The serotonin system has been shown to be important in the neural processing of anxiety and the neurobiological control of learning and memory. Similar to the glutamatergic pathway, altered serotonergic neurotransmission is speculated to contribute to schizophrenia susceptibility. Evidence suggests that METH, which acts as a substrate for the 5-HT transporter, elevates extracellular 5-HT levels by both promoting the efflux via transporter-mediated exchange and by increasing cytoplasmic levels by disrupting storage of 5-HT in vesicles (Cadet and Krasnova 2009; Rothman and Baumann 2003). Although studies have explored whether there is an association between several of the genes involved in 5-HT regulation and MAP, only the rs878567 polymorphism in the 5-HT1A receptor was significantly associated using an alpha level of 0.001 as significant (Kishi et al. 2010). Polymorphisms in the 5-HT1A receptor have been associated with schizophrenia in several studies (Huang et al. 2004; Le Francois et al. 2008; Lemonde et al. 2003, 2004), including the rs878567 polymorphism. Currently, 5-HT(1A) receptor agonists are being considered for the treatment of schizophrenia (McCreary and Jones 2010).
Prokineticin receptor 2 (PROKR2)
PROKR2 encodes prokineticin receptor 2 (PK-R2), an integral membrane G-protein coupled receptor for prokineticins. Prokineticins and their receptors are involved in a wide range of biological functions in multiple organ systems. In the CNS, prokineticin 2 modulates neurogenesis, circadian rhythms, and migration of the subventricular zone-derived neuronal progenitors (Cheng et al. 2002; Ng et al. 2005). The involvement of PK-R2 in circadian rhythm raises the question of whether this gene may be associated with mood disorders, a phenotype often observed in patients with drug addiction. Several animal studies have shown that METH increases expression of circadian genes in the brain (Iijima et al. 2002). The PROKR2 gene is located on chromosome 20p12.3, which was shown to be linked to bipolar disorder in three studies (Detera-Wadleigh et al. 1997; Fanous et al. 2008; Ross et al. 2008). An association between PROKR2 gene variants and major depressive disorder and bipolar disorder was reported in a Japanese population (Kishi et al. 2009b); however, this study was small and confirmatory studies will be necessary to validate this finding. Furthermore, these same investigators have shown that 3 individual SNPs in the PROKR2 gene (rs6085086, rs4815787, rs3746682), as well their haplotypes (p ≤ 0.00019), were associated with MAP (Kishi et al. 2010).
Glycine transporter 1 (SLC6A9)
SLC6A9, which encodes the glycine transporter type 1 (GLYT1), is involved in glutamatergic neurotransmission, particularly at NMDA-type glutamate receptors. It is currently believed that termination of the different synaptic actions of glycine is produced by rapid re-uptake through two sodium- and chloride-coupled transporters, GLYT1 (located in the plasma membrane of glial cells) and GLYT2 (located in pre-synaptic terminals). GLYT1 regulates both glycinergic and glutamatergic neurotransmission by controlling the reuptake of glycine at synapses. The NMDA receptors are regulated in vivo by the amino acids glycine and D-serine. Glycine levels, in turn, are regulated by GLYT1, which serves to maintain low sub-saturating glycine levels in the vicinity of the NMDA receptors. Competitive antagonists of NMDA receptors produce a psychotic state in healthy subjects and exacerbate symptoms in schizophrenics. SLC6A9 has emerged as a key novel target for the treatment of schizophrenia. Morita et al. examined SLC6A9 among Japanese subjects with METH-dependence and psychosis (Morita et al. 2008). Two SNPs conferred an increased risk for MAP (rs2486001 and rs2248829) in addition to the haplotype T-G (p = 0.000037). It is speculated that variants of the SLC6A9 gene may affect susceptibility to MAP by modulating NMDA receptor function.
In summary, there is reasonably strong evidence that genetic variation in neurotransmitter systems and in neural development or growth is associated with risk for MAP. Of the seven genes with strong empirical support for association with MAP, four are involved in glutamatergic neurotransmission (DAOA, DTNBP1, GRM2 and SLC6A9). Potential epistatic (i.e. gene x gene) interactions among these glutamatergic genes should be investigated. Polymorphism in a key gene in serotonin system regulation and signaling (HTR1A) is also associated with risk for MAP, which suggests that other 5-HT system genes may also be good candidates. Finally, two genes with roles in CNS development (FZD3) and neurogenesis (PROKR2) may help to identify other genes as well as developmental processes that mediate vulnerability to MAP.
Gaps in genetic research
These studies reviewed in relation to MAP have examined candidate genes selected based on current concepts of neurobiology. Priorities for future genetic research on MAP include: replicate genetic associations within and across ethnically diverse populations; adjust for multiple comparisons to minimize false-positive associations; utilize linkage disequilibrium and tagSNP information to capture the polymorphic structure of candidate genes; increase statistical power by using larger population cohorts to minimize false-negative associations; improve phenotyping of MAP by use of a continuum versus a categorical classification and account for trajectory of cessation, treatment response and relapse; identification of allele-specific in vivo activity in humans and non-human animals.
METH neuropathobiology
Lines of evidence support the notion that METH abuse leads to neurodegeneration and, as such, may be a component part of MAP pathobiology (Krasnova and Cadet 2009). The neuropsychological events noted show deficits in attention, working memory, and decision making in METH addicts. Bioimaging and histopathologic evaluations show that the clinical findings parallel composite damage to dopamine and serotonin axons, loss of gray matter with linked hypertrophy of the white matter and microgliosis in different brain areas (Kuhn et al. 2008; Thomas et al. 2004a, b, 2008a, b; Xu et al. 2005). The molecular basis of such neurotoxicities, interestingly, parallel neural damage seen in a range of neurodegenerative disorders that include Alzheimer’s and Parkinson’s disease, HIV-associated neurocognitive disorders, and amyotrophic lateral sclerosis. Such neurotoxicity and inevitable neurodegeneration parallels the presence of oxidative stress, excitotoxicity, neuroinflammation, mitochondrial dysfunction, decreased antioxidants and stress patterns (Cadet and Krasnova 2009). These effects a host of intracellular organelle functions and suggests that a range of therapeutic strategies can be developed that would slow or reverse adverse neuronal events (Kosloski et al. 2010; Mosley and Gendelman 2010; Stone et al. 2009).
Animal models
In this section, we review the published preclinical animal research that aims to simulate or directly understand some facet of METH-related psychosis. We will not attempt to survey the vast literature on neurobiologic and neurotoxic effects of METH exposure as it is beyond the scope and the focus of the present paper. The interested reader is referred to the following reviews on these topics (Cadet and Krasnova 2009; Fleckenstein et al. 2007; Volz et al. 2007). This section of the review, however, will have an eye toward the validity of the animal models that have been used to date, as well as identifying key gaps in the methods and research that need to be filled. Given the paucity of animal research directly focused on questions related to MAP, an important goal of this section is to make recommendations for future research that involves the development of translationally-relevant models that are reliable (i.e., reproducible) and predictive (Geyer and Markou 1995).
Clearly, establishing animal models that are predictive of the human phenomenon of interest (MAP in this case) will not happen without experimental situations and associated manipulations that are reproducible and consistent within and across laboratories. We agree with writers that espouse the best way to this broad form of predictive validity and to strong translational animal models is to work toward improving construct and etiological validity (Geyer and Markou 1995; Markou et al. 2009). For construct validity, attention to continually improving the match between what is measured at the behavioral (psychological) and neural levels in the animal models of MAP with what is believed to be the behavioral and neural processes underlying this psychosis in humans (Markou et al. 2009) is essential. As an example, if the current state of knowledge suggests that individuals with MAP have impaired sensorimotor gating that leads to sensory flooding and cognitive fragmentation, then some insight regarding the human condition may come from a better understanding of a similar attentional process in animal models. In this example, pre-pulse inhibition (PPI) may be of particular import. Pre-pulse inhibition refers to the decrease in startle response evoked by an auditory stimulus that is preceded by a pre-pulse stimulus (usually the startle stimulus at lower intensity and shorter duration). Deficits in PPI inhibition have been reported in patients diagnosed with schizophrenia, as well as in rats pretreated with METH (Abekawa et al. 2008) or have a history of self-administering METH (Hadamitzky et al. 2011; see later).
The strategy of searching for and establishing construct validity likely means that no single animal model will be sufficient to capture all processes relevant to the disorder of interest in humans. Also, this strategy is inherently translational. Working toward etiologic validity further encourages the communication and sharing of ideas, theories, and methods necessary for successful translational research (Markou et al. 2009). Etiologic validity refers to the matching of environmental and physiological precursors presumably responsible for the onset of the disorder. There is ongoing debate as to the extent that METH induces psychotic symptoms versus exacerbating pre-existing symptoms (see earlier). This is one of many unique instances in which animal models could serve to inform this important clinical issue. For instance, will rats intravenously self-administer enough METH to alter those behavioral and neural processes altered in humans diagnosed with MAP? Are the predisposing factors, whether environmental, neural, or genetic, thought to be relevant in human MAP contributing to such effects in the animal models? Prior research has demonstrated that under some conditions rats self-administering METH will show deficits in object recognition memory (Reichel et al. 2011) and PPI (Hadamitzky et al. 2011). Unfortunately, this research is quite limited within the context of greater understanding of MAP in humans. Given that METH psychosis is associated with severe behavioral and neuropsychiatric complications (see earlier), there is a pressing need to definitively and precisely identify the behavioral and neurobiological mechanisms underlying the development of METH psychosis. As a result of this identification, behavioral interventions and psychopharmacological treatment strategies can then be developed.
Despite the limited work, animal studies have consistently shown that many psychological processes underlying clinical features of METH psychosis can be reproduced in a variety of animal species, including mice, rats, guinea pigs, cats and non-human primates (e.g., Japanese monkeys). Like METH addicts, animals that are chronically treated with a low (non-toxic) dose of METH gradually develop psychotic-like symptoms, such as a decrease in motor activity, an increase in stereotypies, decreased interest to external stimuli and surroundings, and decreased social functioning (Kuczenski et al. 2009). Also, some of the changed behavioral patterns (e.g., behavioral sensitization) persist even long after the cessation of drug treatment, and have a tendency to relapse or exacerbation upon re-exposure to the drug. Furthermore, concurrent antipsychotic drug treatment can prevent recurrence triggered by METH use (Misra et al. 2000). These results strongly suggests that animal models of METH psychosis have high face (e.g., similarities to clinical symptoms), construct (e.g., METH use and associated brain changes), and predictive validity (e.g., antipsychotic effect), and are effective in reproducing behavioral symptoms of human METH psychosis, mimicking the time course of symptom development, and its liability to exacerbation.
Behavioral characteristics of MAP in nonhuman primates
Most of this work has been carried out by Japanese researchers since the early 1970s beginning during the decade immediately after World War II when METH abuse occurred in epidemic proportions in Japan (Ujike and Sato 2004). Japanese psychiatrists had observed an increased number of cases of MAP in chronic METH users and began to investigate the behavioral characteristics of the psychosis and associated biochemical mechanisms in animals. Nonhuman primate models are thought to be better than other species models in capturing complex and fine-grained behavioral abnormalities resembling human MAP, especially aspects of perceptual aberrations, social interaction, and interpersonal relationship. This is because monkeys, especially Japanese monkeys (Macaca fuscata) are well known to form a stable and intricate hierarchical society in which each member follows a certain rank order appropriate in interacting with others.
An early study provided a vivid description of acute and chronic effects of METH treatment on a group of Japanese monkeys (Machiyama 1992). They gave monkeys intramuscular METH injection at 1.0 mg/kg from Monday to Saturday for 3–6 months. At the same time, physiological saline was given by intramuscular injection to the other animals that served as controls. Upon acute treatment, some monkeys showed motor excitation, whereas other showed motor suppression. They identified that this marked individual difference was due to the different behavioral traits of monkeys in the group. Active monkeys that showed enhanced repetitive motor activity to acute METH were those aggressive individuals occupying higher ranks while non-active monkeys, at the bottom in the ranking order, demonstrated a decrease in motor activity to acute METH treatment. Over the course of repeated drug treatment, some monkeys developed behavioral abnormalities in a variety of psychological domains. In the sensorimotor domain, after about 1 month treatment, some monkeys displayed a stereotypical body-fingering behavior which entailed continuous fingering and investigating certain parts of the body, such as the wrist, thigh, abdomen, penis, or scrotum (Machiyama 1992). In the perception domain, after about 2 months of repeated treatment, some monkey displayed hallucination-like perceptual distortions (e.g., “staring” at vacant space, floor or certain body parts of their own or cage mates, and at times touching with fingers). In the social behavior domain, some monkeys gradually lost interest in social interaction (e.g., grooming and mounting), and withdrew to stay in a location in the cage, and could suddenly show inexplicable aggression and fear, and broken behavioral response patterns (termed “splitting”). There were also marked individual differences. Monkeys, with middle and high ranks that responded actively to acute METH injection, demonstrated the most severe behavioral abnormalities in response to chronic METH treatment. Monkeys with lower ranks fared better and only exhibited mild changes. Two high ranked monkeys even died before the entire experiment was completed. After the termination of daily injections, some behavioral abnormalities disappeared, while others persisted for an extended period. In general, perceptual aberrations (e.g., staring, fixation) diminished rather rapidly and to a greater extent than deficits in sensorimotor (e.g., fixating) and social functioning (e.g., social withdrawal). Also, some monkeys who exhibited persistent behavioral changes that were easily identifiable by uninformed observers recovered better than others. Most interestingly, the psychotic behaviors of chronically treated monkeys could be re-triggered by a saline injection or a METH injection, mimicking clinical phenomena of stress and drug priming-induced relapse of METH psychosis.
One of the issues with these observational studies is the lack of formal assessments of psychological functions. Therefore, it is unclear what psychological function(s) (e.g., attention, working memory, episodic memory, executive functioning, emotional regulation, etc.) were impaired by chronic METH treatment that contributed to the observed behavioral abnormalities. The second issue is that the experimenter-controlled METH administration regimen did not mimic human METH use (via self-administration) and the constant dosing regimen used in the monkey studies also did not reflect the typical pattern of human METH use. Most METH abusers start with very low doses of the drug and have had a long history of progressively escalating their doses. Thus, human METH psychosis often appears during the course of escalating dosage of drug administration (i.e., “binges” or “runs”), and discontinuation of drug usage usually results in a rapid decline of the psychosis, closely paralleling urine drug levels (Angrist 1994; Davis and Schlemmer 1980; Kuczenski et al. 2009). This aspect of METH use pattern was not mimicked in these early studies.
There are several issues that may hinder the effort to delineate the neurobiological underpinnings of METH psychosis. The first is the lack of validated rodent behavioral models of METH psychosis. Unlike non-human primate models, which provide richer behavioral repertoires sensitive to the psychotomimetic effect of METH, most rodent studies focus on motor activity and stereotypy. Behavioral sensitization is taken as signs of METH psychosis (Martinez et al. 2005; Nestler 2001; Robinson and Becker 1986; Segal and Mandell 1974; Segal et al. 1981). One issue with psychomotor sensitization and stereotypy as behavioral indices of METH psychosis is that they do not seem to capture the emotional, cognitive, and perceptual disturbances that characterize human METH psychosis disorders. Other behavioral abnormalities induced by repeated METH treatment, such as disruption of PPI of acoustic startle response, may provide a better model (Braff et al. 2001). The following section discusses this rodent research in more detail.
Behavioral characteristics of METH psychosis in mice and rats
To date, the behavioral models explicitly developed for studying symptoms of MAP in rodents fall into three broad categories: i) METH-induced deficits in PPI, ii) METH attenuation of social interactions, and iii) METH induced stereotypy and alterations in locomotion. The following narrative will provide an overview of this research and highlight some key findings that indicate their possible utility for understanding aspects of MAP in humans.
Pre-pulse inhibition (PPI)
Rodents will startle to a sudden loud sound such as a 40 ms, 120-dB white noise. Under typical conditions, this startle reaction is reduced if this startle stimulus is preceded by a shorter and less intense pre-pulse stimulus (e.g., 20 ms, 72-dB white noise). This inhibition of the startle is thought to measure sensorimotor gating (Braff and Geyer 1990). Individuals diagnosed with schizophrenia show deficits in PPI; an effect that can be simulated by acute and repeated treatment with METH. For example, Arai and colleagues found that mice pretreated with 3 mg/kg METH showed reduced PPI (Arai et al. 2008). A similar acute METH treatment protocol has been shown to be effective at producing PPI deficits in rats (Maehara et al. 2008). Of note, this deficit in PPI was not seen in mice treated acutely with 1 mg/kg METH (Arai et al. 2008). However, in that same report by Arai et al., repeated treatment with METH (1 mg/kg) for 7 days disrupted PPI and this deficit persisted after 7 days of abstinence from METH. More recently, Hadamitzky et al. (2011) report deficits in PPI in rats that had an extended history of self-administering intravenous METH. This research reflects an important advance in developing a translational model to study aspects of MAP. That is, like humans, this alteration in sensorimotor gating was self-induced by the rat.
Behavioral pharmacology research investigating the mechanism of the METH-induced deficits in PPI have focused either on the effects of established antipsychotic medications or on the effects of ligands thought to act on receptor processes involved in aspects of the psychosis. Along the former lines, the typical antipsychotic medication haloperidol has been shown to alleviate PPI deficits induced by acute treatment with METH (Maehara et al. 2008). Further, the atypical antipsychotics olanzapine and risperidone alleviated PPI deficits induced by repeated METH treatment (Abekawa et al. 2008). Arai and colleagues found that the GABAB agonist baclofen alleviated deficits in PPI induced by acute and chronic METH exposure (Arai et al. 2009). Further, the cholinergic system appears to be important for METH-induced deficits in PPI. For example, pretreatment with the muscarinic agonist oxotremorine blocked METH-induced deficits in PPI (Maehara et al. 2008). In this same report, the investigators did not find an effect of the nicotinic agonist nicotine. This result contrasts with (Mizoguchi et al. 2009) who found that pretreatment with nicotine alleviated PPI deficits produced by METH. Further, the nicotinic antagonist dihydro-β-erythroidine (DHβE) and METHyllycaconitine both blocked this ameliorative effect of nicotine indicating a role for α4β2- and α7-containing nicotinic acetylcholine receptors. The most notable difference in protocol between these discrepant reports was that Maehara et al. 2008 used rats; whereas, Mizoguchi et al. 2009 used mice. Regardless, the comorbidity between METH and tobacco use, as well as schizophrenia and tobacco use, makes this an important area for future research.
Social interaction
When a rat is paired with a conspecific, they show a variety of species-specific behaviors. These behaviors include sniffing, grooming, crawling over or under, and nosing (Barnett 1963). Some researchers have suggested that alternations in social interaction resulting from METH exposure may be a useful model to study the paranoid and social anxiety symptoms of humans with MAP (Clemens et al. 2004; Rawson et al. 2002). Previous research has shown that acute METH treatment can alter social interaction. For example, Shinba et al. 1996 found that rats treated with either 0.1 or 1 mg/kg METH on average spent their time further away from the conspecific in the environment than saline controls [see also (Arakawa 1994)]. Perhaps of more interest from a modeling or simulation perspective is the more recent work by Clemens and colleagues showing ‘social withdrawal’ in rats following an abstinence period from METH (Clemens et al. 2007a, b). In one study, Clemens and colleagues administered METH at 2.5 or 5 mg/kg every 2 h for a total of 4 injections. METH increased general activity in the chamber across the entire 7.5 h treatment/measurement period regardless of dose (Clemens et al. 2004). Rats treated with the highest dose of METH (20 mg/kg total) also developed head weaving at the end of the treatment period, suggesting stereotypy with this protocol (see next section). Acute high dose METH increased activity and head-weaving. Albeit interesting, the most notable finding from the perspective of this report is that following 4 weeks of abstinence from METH these rats had significantly lower social interaction score than controls. Subsequently, this social withdrawal finding was extended to a treatment regimen in which rats were administered 8 mg/kg METH once per week for 16 weeks (Clemens et al. 2007a, b). In fact, the alterations in social interaction were seen after 7 weeks of abstinence.
Stereotypy and alterations in locomotion
As the acute dose of METH increases, its behavioral effect in rats and mice generally shifts from inducing hyperactivity to inducing stereotypy. These effects of METH can become exaggerated (i.e., sensitization) with repeated treatment (Bevins and Peterson 2004; Kuczenski et al. 2009; Maehara et al. 2008; Ujike et al. 1989). Further, a handful of papers published in the 1960s showed that spontaneous wheel running, activity, and reactivity to external stimuli are blunted long after (e.g., 10 weeks) chronic treatment with METH has ceased [see Utena 1961, 1966); Yagi 1963; Yui et al. 2000 and Machiyama 1992 for more detailed discussion of this and related research]. The presentation of such behavioral alterations that sensitize with repeated treatment and persist with abstinence has been considered a model for some of the symptoms of psychosis (Machiyama 1992; Takigawa et al. 1993; Yui et al. 2000). Converging support for this notion comes from the overlap in the neurobiological processes underlying these motor effects of METH and that of psychosis in humans, as well as behavioral pharmacologic work indicating the effectiveness of antipsychotic medications. For instance, METH-induced locomotor stimulation is blocked by clozapine, haloperidol, and chlorpromazine (Maehara et al. 2008; Okuyama et al. 1997). Notably, METH-induced stereotypy in the work of Okuyama et al. 1997 was blocked by haloperidol and chlorpromazine, but not clozapine. Additional pharmacologic research has implicated the serotonergic, dopaminergic, and muscarinic systems in these effects (Balsara et al. 1979; Maehara et al. 2008; Ujike et al. 1989). For instance, Ujike et al. 1989 found that repeated daily METH treatment in rats (4 mg/kg for 14 days) increased locomotion and stereotypy. Pretreatment with dopamine D1 receptor antagonists SCH23390 or the D2 receptor antagonist YM-09151-2 blocked this sensitization.
Most of the repeated or chronic METH exposure research in this area involves a daily injection of METH for a prescribed number of days. Notably, a recent study by Kuczenski et al. (2009) sought to investigate these shifts in locomotor stimulation and stereotypy in rats, using an intravenous infusion protocol designed to more closely mimic the chronic escalating use of METH seen in humans and simulate the longer half-life of METH in humans (ca. 12 h in human versus 1 h in rats). Their findings confirm and extend earlier research describing the development of stereotypy, as well as disruption of sleep, in what seems to be a more translationally relevant model of MAP. Whether this is the case or not will need to await further research. However, one limitation of this model that will need to be overcome, if this model is to be more widely adopted, is the high mortality rate of the rats in the escalating METH exposure phase.
Neurobiological mechanisms underlying MAP
Chronic METH use profoundly changes the brain structures and chemistry (Chang et al. 2007). Studies on the METH-induced brain changes relevant to MAP have focused on the catecholaminergic systems (e.g., dopamine, norepinephrine, 5-HT, etc.). This approach makes sense given that METH is known to cause both acute and chronic neurotoxic changes in dopaminergic and serotonergic neurons in animals and humans and that psychosis in schizophrenia is thought to result from hyperactivity of the mesolimbic dopaminergic system. Some structural changes (enlarged lateral ventricle, enlarged basal ganglia, reduced hippocampal volume), that have been reported in patients with schizophrenia, have also been found in people who were chronic METH users, strongly indicating that these structural changes and their underlying mechanisms may be responsible for METH psychosis (Chang et al. 2007).
Human postmortem and imaging studies have consistently shown that chronic METH use causes a reduction of dopamine transporter (DAT) density in the various dopaminergic systems, including the dorsal striatum, nucleus accumbens, and prefrontal cortex. However, this pronounced effect persists long after cessation of drug administration (Sekine et al. 2001, 2003; Volkow et al. 2001c; Wilson et al. 1996). The striatal dopamine D2 density is generally not affected. However, lower level of D2 receptor availability in the orbitofrontal cortex has been reported (Volkow et al. 2001a). Furthermore, there seem to be a significant negative correlation between the clinical severity of psychotic symptoms and DAT density, and a positive correlation between the duration of METH use and the severity of psychotic symptoms, suggesting that chronic METH use causes the reduction in DAT density, which may directly contribute to the development of METH psychosis (Iyo et al. 2004). Volkow et al. (2001b) also reported that the chronic METH-induced reduction in DAT is associated with motor and cognitive impairment. They found that there was a negative correlation between the DAT reductions and the impaired motor performance, and a positive correlation between the levels of DAT reductions and verbal memory performance: the lower the dopamine (DA) transporter level, the slower the motor responses, and the greater worsening of memory performance. However, because the reduced striatal DAT density tends to recover following METH abstinence (>6 months) (Volkow et al. 2001b) while METH psychosis persists long after the absence of METH use (Sato 1992), the direct and definite link between DAT reduction and METH psychosis is still lacking. It is possible that other permanent neuroadaptions caused by DAT reduction also play a role. Other human studies also reported that during the spontaneous recurrences of METH psychosis (referred to as “flashbacks”), there were increases in plasma DA and norepinephrine levels (Yui et al. 1999), consistent with the notion that increased DA neurotransmission, coupled with increased noradrenergic hyperactivity (due to stress) may contribute to psychotic symptoms.
In contrast to limited research on human subjects, there are many studies that have examined METH-induced brain changes in rodents. Although many structural and neurochemical changes induced by METH use/abuse has been revealed, whether those changes are directly related to MAP or even contributing to MAP is less certain. To ensure that the neurochemical changes discussed here are potentially relevant to the underpinnings of MAP, in this section, we will narrow our discussion to research that has demonstrated behavioral sensitization following human patterns of METH abuse (e.g., chronic and escalating dose regimens followed by repeated high-dose METH use). Behavioral sensitization refers to a progressive increase in motor and stereotypical responses to repeated drug treatment (Martinez et al. 2005; Nestler 2001; Robinson and Becker 1986). Thus, the acute effect of METH use is not covered here. The rationale behind this approach is that endogenous sensitized dopaminergic function (e.g., progressively enhanced DA supersensitivity) is believed to be the critical cause of MAP (Ujike 2002; Ujike and Sato 2004). Because behavioral sensitization is the direct “readout” of central DA sensitization, it becomes an indispensable proxy measure of MAP. Because clinical observations suggest that MAP (as well as other psychostimulant psychosis) often emerges after repeated high-dose binges or runs, typically preceded by a more intermittent and escalating pattern of METH abuse, animal models that utilize a long-term intermittent and/or escalating dose regimen, followed by repeated high-dose METH runs are most likely to capture the clinical feature of MAP, and afford us the best chance to identify the neurochemical changes accompanied with MAP. Thus, studies utilizing this approach will be emphasized.
Chronic treatment of METH causes an increase of dopaminergic neurotransmission in the striatum. For example, Nishikawa et al. (1983) found that repeated METH treatment (6 mg/kg per day for 3–14 days) produced a robust behavioral sensitization in the form of augmented stereotypy. A later challenge injection of a lower METH dose (2 mg/kg) increased DA turnover (lower DA and higher 3, 4-dihydroxyphenylacetic acid levels, higher ratios of 3, 4-dihydroxyphenylacetic acid over DA) in the striatum and mesolimbic area of the sensitized animals. Kazahaya et al. (1989) also found that a challenge injection of METH after 7 days withdrawal from chronic administration of METH (4 mg/kg for 14 days) markedly increased DA release in the striatum. Segal and Kuczenski (1997) found that multiple short-interval METH injections (four daily injections at 2-hr intervals at 4.42 mg/kg) produced an augmented DA and serotonin release in both the nucleus accumbens and caudate-putamen in rats that were pretreated with METH for 16 days. These findings, coupled with the findings that repeated METH treatment directly sensitizes DA receptors in the nucleus accumbens and ventral tegmental area (Amano et al. 1996, 2003), and reduces striatal DAT (Izquierdo et al. 2010), strongly suggests that the enhanced striatal DA neurotransmission (e.g. increased DA release and supersensitivity of receptors) plays an important role in METH-induced behavioral sensitization. As discussed earlier, pharmacologic evidence is consistent with this finding. Ujike et al. 1989 showed that pretreatment of SCH 23390 (a selective D1 DA receptor antagonist) or YM-09151-2 (a selective D2 DA receptor antagonist) prevented the development of behavioral sensitization induced by repeated METH injection for 14 days and reduced the enhanced DA release produced by METH challenge.
Chronic METH users often take the drug continuously, and the plasma drug concentrations are elevated and maintained throughout a 2–3 day binge. Studies simulating this pattern of human METH use often employ an escalating dose (ED)-multiple binge rodent model (Kuczenski et al. 2009; Segal and Kuczenski 1997, 1999). Kuczenski et al. (2009) developed a computer-controlled, intravenous drug delivery methodology with dynamic infusion which imposes a 12-hour half-life of the drug in rats to reproduce a plasma METH profile that approximates human METH pharmacokinetics. Using this drug administration procedure, they still found a prolonged elevation in caudate extracellular DA and behavioral sensitization.
In addition to DA, chronic METH use also produces changes in other neurotransmitter systems, which are also implicated in METH-induced behavioral sensitization. For example, it has been shown that repeated METH treatment (3 mg/kg/day for 30–50 days) increased serotonin levels in cats, which were restored by chlorpromazine treatment (Utena 1966). Rats receiving single daily injections of METH, followed by multiple runs (four daily injections at 2-hr intervals) showed a decrease in serotonin response in the striatum during runs (Segal and Kuczenski 1997). The findings that pretreatment with MDL 72222, a 5-HT3 antagonist, can attenuate both the development and expression of METH-induced behavioral sensitization (Yoo et al. 2006), and repeated treatment with aripiprazole (a drug with 5-HT1A agonist action) during the withdrawal period from repeated METH treatment attenuated METH-induced behavioral sensitization (Futamura et al. 2010), also point to the notion that serotonin-mediated neurotransmission are related to the psychotomimetic effect of METH. Other neurotransmitters implicated in METH sensitization include substance P and sigma receptors, as evidenced by the findings that repeated METH treatment decreased the substance P receptor binding (Ujike et al. 1988) and sigma receptor antagonist BMY 14802 prevents the development of behavioral sensitization induced by repeated administration of METH (Ujike et al. 1992).
Conclusion
There is great need for increased research to further understand factors related to MAP and how this psychosis affects METH use, dependence, and treatment outcomes. The current state of knowledge suggests that these factors, and their interaction, will span genetic to socioenvironmental influences. As such, research directed at the better understanding of MAP, by necessity, will need to be translational. This review identifies some gaps in our understanding and outlined potential future avenues of research that could help realize this goal of better treatment efficacy for METH-dependent individuals with MAP. For instance, there are no studies assessing protective factors for MAP, which may mitigate against risk factors for psychosis. Does level of familial support, as an example, during treatment affect symptom expression and/or treatment outcome? Along these lines, there is a solid foundation of genetics work that has identified several candidate genes that may play a role in MAP development. However, larger studies of more ethnically diverse populations that likely have unique risk and protective factors are needed. Finally, advances in the development of animal models that show etiological and predictive validity are needed for a more complete understanding of the causes of MAP. These models would likely assist in drug development and behavioral interventions.
References
Abekawa T, Ito K, Nakagawa S, Nakato Y, Koyama T (2008) Olanzapine and risperidone block a high dose of methamphetamine-induced schizophrenia-like behavioral abnormalities and accompanied apoptosis in the medial prefrontal cortex. Schizophr Res 101:84–94
Akiyama K (2006) Longitudinal clinical course following pharmacological treatment of methamphetamine psychosis which persists after long-term abstinence. Ann New York Acad Sci 1074:125–134
Amano T, Matsubayashi H, Sasa M (1996) Hypersensitivity of nucleus accumbens neurons to methamphetamine and dopamine following repeated administrations of methamphetamine. Ann N Y Acad Sci 801:136–147
Amano T, Matsubayashi H, Seki T, Sasa M, Sakai N (2003) Repeated administration of methamphetamine causes hypersensitivity of D2 receptor in rat ventral tegmental area. Neurosci Lett 347:89–92
Angrist B (1994) Amphetamine psychosis: clinical variations of the syndrome. In: Cho A, Segal D (eds) Amphetamine and its analogues. Academic, San Diego, pp 387–414
Aoyama N, Takahashi N, Kitaichi K, Ishihara R, Saito S, Maeno N et al (2006) Association between gene polymorphisms of SLC22A3 and methamphetamine use disorder. Alcohol Clin Exp Res 30:1644–1649
APA American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders, 4th edn. American Psychiatric Association, Washington
Arai S, Takuma K, Mizoguchi H, Ibi D, Nagai T, Takshashi K et al (2008) Involvement of pallidotegmental neurons in methamphetamine- and MK-801-induced impairment of prepulse inhibition of the acoustic startle reflex in mice: reversal by GABAB receptor agonist baclofen. Neuropsychopharmacology 33:3164–3175
Arai S, Takuma K, Mizoguchi H, Ibi D, Nagai T, Kamei H et al (2009) GABAB receptor agonist baclofen improves methamphetamine-induced cognitive deficit in mice. Eur J Pharmacol 602:101–104
Arakawa O (1994) Effects of methamphetamine and methylphenidate on single and paired rat open-field behavior. Physiol Behav 55:441–446
Arseneault L, Cannon M, Witton J, Murray RM (2004) Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry 184:110–117
Badner JA, Gershon ES (2002) Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry 7:405–411
Balsara JJ, Jadhav JH, Muley MP, Chandorkar AG (1979) Effects of drugs influencing central serotonergic mechanisms on methamphetamine stereotyped behavior in the rat. Psychopharmacology 64:303–307
Barnett SA (1963) The rat: a study in behaviour. Aldine, Chicago
Batki SL, Harris DS (2004) Quantitative drug levels in stimulant psychosis: relationship to symptom severity, catecholamines and hyperkinesias. Am J Addict 13:461–470
Benson MA, Newey SE, Martin-Rendon E, Hawkes R, Blake DJ (2001) Dysbindin, a novel coiled-coil-containing protein that interacts with the dystrobrevins in muscle and brain. J Biol Chem 276:24232–24241
Bevins RA, Peterson JL (2004) Individual differences in rats’ reactivity to novelty and the unconditioned and conditioned locomotor effects of methamphetamine. Pharmacol Biochem Behav 79:65–74
Blouin JL, Dombroski BA, Nath SK, Lasseter VK, Wolyniec PS, Nestadt G et al (1998) Schizophrenia susceptibility loci on chromosomes 13q32 and 8p21. Nat Genet 20:70–73
Braff DL, Geyer MA (1990) Sensorimotor gating and schizophrenia: human and animal model studies. Arch Gen Psychiatry 47:181–188
Braff DL, Geyer MA, Light GA, Sprock J, Perry W, Cadenhead KS et al (2001) Impact of prepulse characteristics on the detection of sensorimotor gating deficits in schizophrenia. Schizophr Res 49:171–178
Cadet JL, Krasnova IN (2009) Molecular bases of methamphetamine-induced neurodegeneration. Int Rev Neurobiol 88:101–119
Cartmell J, Monn JA, Schoepp DD (1999) The metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus d-amphetamine motor behaviors in rats. J Pharmacol Exp Ther 291:161–170
Caton CLM, Drake RE, Hasin DS, Domingues B, Shrout PE, Samet S et al (2005) Differences between early-phase primary psychotic disorders with concurrent substance use and substance-induced psychoses. Arch Gen Psychiatry 52:137–145
Chang L, Alicata D, Ernst T, Volkow N (2007) Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction 102(Suppl 1):16–32
Chen CK, Lin SK, Sham PC, Ball D, Loh EW, Hsiao CC et al (2003) Pre-morbid characteristics and co-morbidity of methamphetamine users with and without psychosis. Psychol Med 33:1407–1414
Chen CK, Hu X, Lin SK, Sham PC, Loh EW, Li T et al (2004) Association analysis of dopamine D2-like receptor genes and methamphetamine abuse. Psychiatr Genet 14:223–226
Chen CK, Lin SK, Sham PC, Ball D, Loh EW, Murray RM (2005) Morbid risk for psychiatric disorder among the relatives of methamphetamine users with and without psychosis. Am J Med Genet B Neuropsychiatr Genet 136:87–91
Chen CK, Lin SK, Huang MC, Su LW, Hsiao CC, Chiang YL et al (2007) Analysis of association of clinical correlates and 5-HTTLPR polymorphism with suicidal behavior among Chinese methamphetamine abusers. Psychiatry Clin Neurosci 61:479–486
Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J et al (2002) Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature 417:405–410
Christian DR, Huber A, Brecht M-L, McCann MJ, Marinelli-Casey P, Lord RH et al (2007) Methamphetamine users entering treatment: characteristics of the methamphetamine treatment project sample. Subst Use Misuse 42:2207–2222
Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, Abderrahim H et al (2002) Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc Natl Acad Sci USA 99:13675–13680
Clemens KJ, van Nieuwenhuyzen PS, Li KM, Cornish JL, Hunt GE, McGregor IS (2004) MDMA (“ecstasy”), methamphetamine and their combination: long-term changes in social interaction and neurochemistry in the rat. Psychopharmacology 173:318–325
Clemens KJ, Cornish JL, Hunt GE, McGregor IS (2007a) Repeated weekly exposure to MDMA, methamphetamine or their combination: long-term behavioural and neurochemical effects in rats. Drug Alcohol Depend 86:183–190
Clemens KJ, McGregor IS, Hunt GE, Cornish JL (2007b) MDMA, methamphetamine and their combination: possible lessons for party drug users from recent preclinical research. Drug Alcohol Rev 26:9–15
Cotter D, Kerwin R, Al-Sarraji S, Brion JP, Chadwich A, Lovestone S et al (1998) Abnormalities of Wnt signalling in schizophrenia–evidence for neurodevelopmental abnormality. Neuroreport 9:1379–1383
Davis JM, Schlemmer FP Jr (1980) The amphetamine psychosis. In: Caldwell J (ed) Amphetamines and related stimulants: chemical, biological, clinical and social aspects. CRC Press, Boca Raton, pp 161–173
Detera-Wadleigh SD, Badner JA, Yoshikawa T, Sanders AR, Goldin LR, Turner G et al (1997) Initial genome scan of the NIMH genetics initiative bipolar pedigrees: chromosomes 4, 7, 9, 18, 19, 20, and 21q. Am J Med Genet 74:254–262
Edwards TL, Wang X, Chen Q, Wormly B, Riley B, O’Neill FA et al (2008) Interaction between interleukin 3 and dystrobrevin-binding protein 1 in schizophrenia. Schizophr Res 106:208–217
Ezaki N, Nakamura K, Sekine Y, Thanseem I, Anitha A, Iwata Y et al (2008) Short allele of 5-HTTLPR as a risk factor for the development of psychosis in Japanese methamphetamine abusers. Ann N Y Acad Sci 1139:49–56
Fanous AH, Neale MC, Webb BT, Straub RE, O’Neill FA, Walsh D et al (2008) Novel linkage to chromosome 20p using latent classes of psychotic illness in 270 Irish high-density families. Biol Psychiatry 64:121–127
Fergusson DM, Horwood LF, Swain-Campbell NR (2003) Cannabis dependence and psychotic symptoms in young people. Psychol Med 33:15–21
Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR (2007) New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol 47:681–698
Fujii D (2002) Risk factors for treatment-resistive methamphetamine psychosis. J Neuropsychiatry Clin Neurosci 14(2):239–240
Futamura T, Akiyama S, Sugino H, Forbes A, McQuade RD, Kikuchi T (2010) Aripiprazole attenuates established behavioral sensitization induced by methamphetamine. Prog Neuropsychopharmacol Biol Psychiatry 34:1115–1119
Geyer MA, Markou A (1995) Animal models of psychiatric disorders. In: Bloom FE, Kupfer DJ (eds) Psychopharmacology: the fourth generation of progress. Raven, New York, pp 787–798
Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, Hillhouse M, Ang A, Rawson R et al (2008) Clinical course and outcomes of methamphetamine-dependent adults with psychosis. J Subst Abuse Treat 35:445–450
Grant KM, Kelley SS, Agrawal S, Meza JL, Meyer JR, Romberger DJ (2007) Methamphetamine use in rural midwesterners. Am J Addict 16:79–84
Gurling HM, Kalsi G, Brynjolfson J, Sigmundsson T, Sherrington R, Mankoo BS et al (2001) Genomewide genetic linkage analysis confirms the presence of susceptibility loci for schizophrenia, on chromosomes 1q32.2, 5q33.2, and 8p21-22 and provides support for linkage to schizophrenia, on chromosomes 11q23.3-24 and 20q12.1-11.23. Am J Hum Genet 68:661–673
Hadamitzky M, Markou A, Kuczenski R (2011) Extended access to methamphetamine self-administration affects sensorimotor gating in rats. Behav Brain Res 217:386–390
Harris D, Batki SL (2000) Stimulant psychosis: symptom profile and acute clinical course. Am J Addict 9:28–37
Harrison PJ, Lyon L, Sartorius LJ, Burnet PW, Lane TA (2008) The group II metabotropic glutamate receptor 3 (mGluR3, mGlu3, GRM3): expression, function and involvement in schizophrenia. J Psychopharmacol 22:308–322
Hashimoto T, Hashimoto K, Matsuzawa D, Shimizu E, Sekine Y, Inada T et al (2005) A functional glutathione S-transferase P1 gene polymorphism is associated with methamphetamine-induced psychosis in Japanese population. Am J Med Genet B Neuropsychiatr Genet 135B:5–9
Huang YY, Battistuzzi C, Oquendo MA, Harkavy-Friedman J, Greenhill L, Zalsman G et al (2004) Human 5-HT1A receptor C(−1019)G polymorphism and psychopathology. Int J Neuropsychopharmacol 7:441–451
Ide S, Kobayashi H, Tanaka K, Ujike H, Sekine Y, Ozaki N et al (2004) Gene polymorphisms of the mu opioid receptor in methamphetamine abusers. Ann N Y Acad Sci 1025:316–324
Ide S, Kobayashi H, Ujike H, Ozaki N, Sekine Y, Inada T et al (2006) Linkage disequilibrium and association with methamphetamine dependence/psychosis of mu-opioid receptor gene polymorphisms. Pharmacogenomics J 6:179–188
Iijima M, Nikaido T, Akiyama M, Moriya T, Shibata S (2002) Methamphetamine-induced, suprachiasmatic nucleus-independent circadian rhythms of activity and mPer gene expression in the striatum of the mouse. Eur J Neurosci 16:921–929
Ikeda M, Iwata N, Suzuki T, Kitajima T, Yamanouchi Y, Kinoshiya Y et al (2006) Positive association of AKT1 haplotype to Japanese methamphetamine use disorder. Int J Neuropsychopharmacol 9:77–81
Ikeda M, Ozaki N, Suzuki T, Kitajima T, Yamanouchi Y, Kinoshita Y et al (2007) Possible association of beta-arrestin 2 gene with methamphetamine use disorder, but not schizophrenia. Genes Brain Behav 6:107–112
Inada T, Iijima Y, Uchida N, Maeda T, Iwashita S, Ozaki N et al (2004) No association found between the type 1 sigma receptor gene polymorphisms and methamphetamine abuse in the Japanese population: a collaborative study by the Japanese Genetics Initiative for Drug Abuse. Ann N Y Acad Sci 1025:27–33
Itoh K, Hashimoto K, Shimizu E, Sekine Y, Ozaki N, Inada T et al (2005) Association study between brain-derived neurotrophic factor gene polymorphisms and methamphetamine abusers in Japan. Am J Med Genet B Neuropsychiatr Genet 132B:70–73
Iwanami A, Sugiyama A, Kuroki N, Toda S, Kato N, Nakatani Y et al (1994) Patients with methamphetamine psychosis admitted to a psychiatric hospital in Japan. Acta Psychiatr Scand 89:428–432
Iwata N, Inada T, Harano M, Komiyama T, Yamada M, Sekine Y et al (2004) No association is found between the candidate genes of t-PA/plasminogen system and Japanese methamphetamine-related disorder: a collaborative study by the Japanese Genetics Initiative for Drug Abuse. Ann N Y Acad Sci 1025:34–38
Iyo M, Sekine Y, Mori N (2004) Neuromechanism of developing methamphetamine psychosis: a neuroimaging study. Ann N Y Acad Sci 1025:288–295
Izquierdo A, Belcher AM, Scott L, Cazares VA, Chen J, O’Dell SJ et al (2010) Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology 35:505–514
Jeong SH, Joo EJ, Ahn YM, Lee KY, Kim YS (2006) Investigation of genetic association between human Frizzled homolog 3 gene (FZD3) and schizophrenia: results in a Korean population and evidence from meta-analysis. Psychiatry Res 143:1–11
Joo A, Shibata H, Ninomiya H, Kawasaki H, Tashiro N, Fukumaki Y (2001) Structure and polymorphisms of the human metabotropic glutamate receptor type 2 gene (GRM2): analysis of association with schizophrenia. Mol Psychiatry 6:186–192
Kanahara N, Miyatake R, Sekine Y, Inada T, Ozaki N, Iwata N et al (2009) Association study between the PIK4CA gene and methamphetamine use disorder in a Japanese population. Am J Med Genet B Neuropsychiatr Genet 150B:233–238
Kantrowitz JT, Malhotra AK, Cornblatt B, Silipo G, Balla A, Suckow RF et al (2010) High dose D-serine in the treatment of schizophrenia. Schizophr Res 121:125–130
Kazahaya Y, Akimoto K, Otsuki S (1989) Subchronic methamphetamine treatment enhances methamphetamine- or cocaine-induced dopamine efflux in vivo. Biol Psychiatry 25:903–912
Kew JN, Pflimlin MC, Kemp JA, Mutel V (2002) Differential regulation of synaptic transmission by mGlu2 and mGlu3 at the perforant path inputs to the dentate gyrus and CA1 revealed in mGlu2 −/− mice. Neuropharmacology 43:215–221
Kinoshita Y, Ikeda M, Ujike H, Kitajima T, Yamanouchi Y, Aleksic B et al (2008) Association study of the calcineurin A gamma subunit gene (PPP3CC) and methamphetamine-use disorder in a Japanese population. Ann N Y Acad Sci 1139:57–62
Kishi T, Ikeda M, Kitajima T, Yamanouchi Y, Kinoshita Y, Kawashima K et al (2008) Prostate apoptosis response 4 gene is not associated with methamphetamine-use disorder in the Japanese population. Ann N Y Acad Sci 1139:83–88
Kishi T, Ikeda M, Kitajima T, Yamanouchi Y, Kinoshita Y, Kawashima K et al (2009a) A functional polymorphism in estrogen receptor alpha gene is associated with Japanese methamphetamine induced psychosis. Prog Neuropsychopharmacol Biol Psychiatry 33:895–898
Kishi T, Tsunoka T, Ikeda M, Kawashima K, Okochi T, Kitajima T et al (2009b) Serotonin 1A receptor gene and major depressive disorder: an association study and meta-analysis. J Hum Genet 54:629–633
Kishi T, Tsunoka T, Ikeda M, Kitajima T, Kawashima K, Okochi T et al (2009c) Serotonin 1A receptor gene is associated with Japanese methamphetamine-induced psychosis patients. Neuropharmacology 58:452–456
Kishi T, Kitajima T, Tsunoka T, Okumura T, Okochi T, Kawashima K et al (2010) PR0KR2 is associated with methamphetamine dependence in the Japanese population. Prog Neuropsychopharmacol Biol Psychiatry 34:1033–1036
Kishimoto M, Ujike H, Motohashi Y, Tanaka Y, Okahisa Y, Kotaka T et al (2008a) The dysbindin gene (DTNBP1) is associated with methamphetamine psychosis. Biol Psychiatry 63:191–196
Kishimoto M, Ujike H, Okahisa Y, Kotaka T, Takaki M, Kodama M et al (2008b) The Frizzled 3 gene is associated with methamphetamine psychosis in the Japanese population. Behav Brain Funct 4:37
Kobayashi H, Hata H, Ujike H, Harano M, Inada T, Komiyama T et al (2006) Association analysis of delta-opioid receptor gene polymorphisms in methamphetamine dependence/psychosis. Am J Med Genet B Neuropsychiatr Genet 141B:482–486
Kobayashi H, Ide S, Hasegawa J, Ujike H, Sekine Y, Ozaki N et al (2004) Study of association between alpha-synuclein gene polymorphism and methamphetamine psychosis/dependence. Ann N Y Acad Sci 1025:325–334
Koizumi H, Hashimoto K, Kumakiri C, Shimizu E, Sekine Y, Ozaki N et al (2004) Association between the glutathione S-transferase M1 gene deletion and female methamphetamine abusers. Am J Med Genet B Neuropsychiatr Genet 126B:43–45
Konradi C, Heckers S (2003) Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment. Pharmacol Ther 97:153–179
Kosloski LM, Ha DM, Hutter JA, Stone DK, Pichler MR, Reynolds AD et al (2010) Adaptive immune regulation of glial homeostasis as an immunization strategy for neurodegenerative diseases. J Neurochem 114:1261–1276
Kotaka T, Ujike H, Morita Y, Kishimoto M, Okahisa Y, Inada T et al (2008) Association study between casein kinase 1 epsilon gene and methamphetamine dependence. Ann N Y Acad Sci 1139:43–48
Kotaka T, Ujike H, Okahisa Y, Takaki M, Nakata K, Kodama M et al (2009) G72 gene is associated with susceptibility to methamphetamine psychosis. Prog Neuropsychopharmacol Biol Psychiatry 33:1046–1049
Krasnova IN, Cadet JL (2009) Methamphetamine toxicity and messengers of death. Brain Res Rev 60:379–407
Kuczenski R, Segal DS, Melega WP, Lacan G, McCunney SJ (2009) Human methamphetamine pharmacokinetics simulated in the rat: behavioral and neurochemical effects of a 72-h binge. Neuropsychopharmacology 34:2430–2441
Kuhn DM, Francescutti-Verbeem DM, Thomas DM (2008) Dopamine disposition in the presynaptic process regulates the severity of methamphetamine-induced neurotoxicity. Ann N Y Acad Sci 1139:118–126
Lambert D, Gale JA, Hartley D (2008) Substance abuse by youth and young adults in rural America. J Rural Health 24:221–228
Le Francois B, Czesak M, Steubl D, Albert PR (2008) Transcriptional regulation at a HTR1A polymorphism associated with mental illness. Neuropharmacology 55:977–985
Leamon MH, Flower K, Salo RE, Nordahl TE, Kranzler HR, Galloway GP (2010) Methamphetamine and paranoia: the methamphetamine experience questionnaire. Am J Addict 19:155–168
Leelahanaj T, Kongsakon R, Netrakom P (2005) A 4-week, double-blind comparison of olanzapine with haloperidol in the treatment of amphetamine psychosis. J Med Assoc Thai 88(suppl 3):S43–S52
Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD et al (2003) Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci 23:8788–8799
Lemonde S, Du L, Bakish D, Hrdina P, Albert PR (2004) Association of the C(−1019)G 5-HT1A functional promoter polymorphism with antidepressant response. Int J Neuropsychopharmacol 7:501–506
Li T, Chen CK, Hu X, Ball D, Lin SK, Chen W et al (2004) Association analysis of the DRD4 and COMT genes in methamphetamine abuse. Am J Med Genet B Neuropsychiatr Genet 129B:120–124
Li W, Zhang Q, Oiso N, Novak EK, Gautam R, O’brien EP et al (2003) Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1). Nat Genet 35:84–89
Liu HC, Lin SK, Liu SK, Chen SL, Hu CJ, Chang JG et al (2004) DAT polymorphism and diverse clinical manifestations in methamphetamine abusers. Psychiatr Genet 14:33–37
Liu HC, Chen CK, Leu SJ, Wu HT, Lin SK (2006) Association between dopamine receptor D1 A-48 G polymorphism and methamphetamine abuse. Psychiatry Clin Neurosci 60:226–231
Lucas FR, Salinas PC (1997) WNT-7a induces axonal remodeling and increases synapsin I levels in cerebellar neurons. Dev Biol 192:31–44
Lyon L, Kew JN, Corti C, Harrison PJ, Burnet PW (2008) Altered hippocampal expression of glutamate receptors and transporters in GRM2 and GRM3 knockout mice. Synapse 62:842–850
Machiyama Y (1992) Chronic methamphetamine intoxication model of schizophrenia in animals. Schizophr Bull 18:107–113
Maehara S, Hikichi H, Satow A, Okuda S, Ohta H (2008) Antipsychotic property of a muscarinic receptor agonist in animal models of schizophrenia. Pharmacol Biochem Behav 91:140–149
Mahoney JJ 3rd, Kalechstein AD, De La Garza R 2nd, Newton TF (2008) Presence and persistence of psychotic symptoms in cocaine- versus methamphetamine-dependence participants. Am J Addict 17:83–98
Manschreck TC, Laughery JA, Weisstein CC, Allen D, Humblestone B, Neville M et al (1988) Characteristics of freebase cocaine psychosis. Yale J Biol Med 61:115–122
Markou A, Chiamulera C, Geyer MA, Tricklebank M, Steckler T (2009) Removing obstacles in neuroscience drug discovery: the future path for animal models. Neuropsychopharmacology 34:74–89
Martinez V, Parikh V, Sarter M (2005) Sensitized attentional performance and Fos-immunoreactive cholinergic neurons in the basal forebrain of amphetamine-pretreated rats. Biol Psychiatry 57:1138–1146
Matsumoto T, Karmijo A, Miyakawa T, Endo K, Yabana T, Kishimoto H et al (2002) Methamphetamine in Japan: the consequences of methamphetamine abuse as a function of route of administration. Addiction 97:809–817
Matsuzawa D, Hashimoto K, Miyatake R, Shirayama Y, Shimizu E, Maeda K et al (2007) Identification of functional polymorphisms in the promoter region of the human PICK1 gene and their association with methamphetamine psychosis. Am J Psychiatry 164:1105–1114
Mccreary AC, Jones CA (2010) Antipsychotic medication: the potential role of 5-HT(1A) receptor agonism. Curr Pharm Des 16:516–521
McKetin R, McLaren J, Lubman DI, Hides L (2006) The prevalence of psychotic symptoms among methamphetamine users. Addiction 101:1473–1478
McKetin R, Ross J, Kelly E, Baker A, Lee N, Lubman DI et al (2008) Characteristics and harms associated with injecting versus smoking methamphetamine among methamphetamine treatment entrants. Drug Alcohol Rev 27:277–285
Misra LK, Kofoed L, Oesterheld JR, Richards GA (2000) Olanzapine treatment of methamphetamine psychosis. J Clin Psychopharmacol 20:393–394
Mizoguchi H, Arai S, Koike H, Ibi D, Kamei H, Nabeshima T et al (2009) Therapeutic potential of nicotine for methamphetamine-induced impairment of sensorimotor gating: involvement of pallidotegmental neurons. Psychopharmacology 207:235–243
Moos RH, Brennan PL, Mertens JR (1994) Diagnostic subgroups and predictors of one-year re-admission among late-middle-aged and older substance abuse patients. J Stud Alcohol 55:173–183
Morio A, Ujike H, Nomura A, Tanaka Y, Morita Y, Otani K et al (2006) No association between CART (cocaine- and amphetamine-regulated transcript) gene and methamphetamine dependence. Ann N Y Acad Sci 1074:411–417
Morita Y, Ujike H, Tanaka Y, Uchida N, Nomura A, Ohtani K et al (2005a) A nonsynonymous polymorphism in the human fatty acid amide hydrolase gene did not associate with either methamphetamine dependence or schizophrenia. Neurosci Lett 376:182–187
Morita Y, Ujike H, Tanaka Y, Uchida N, Nomura A, Otani K et al (2005b) The X-box binding protein 1 (XBP1) gene is not associated with methamphetamine dependence. Neurosci Lett 383:194–198
Morita Y, Ujike H, Tanaka Y, Kishimoto M, Okahisa Y, Kotaka T et al (2008) The glycine transporter 1 gene (GLYT1) is associated with methamphetamine-use disorder. Am J Med Genet B Neuropsychiatr Genet 147B:54–58
Mosley RL, Gendelman HE (2010) Control of neuroinflammation as a therapeutic strategy for amyotrophic lateral sclerosis and other neurodegenerative disorders. Exp Neurol 222:1–5
Nakama H, Chang L, Cloak C, Jiang C, Alicata D, Haning W (2008) Association between psychiatric symptoms and craving in methamphetamine users. Am J Addict 17:441–446
Nakamura K, Chen CK, Sekine Y, Iwata Y, Anitha A, Loh El W et al (2009) An association study of monoamine oxidase A (MAOA) gene polymorphism in methamphetamine psychosis. Neurosci Lett 455:120–123
Nestler EJ (2001) Molecular neurobiology of addiction. Am J Addict 10:201–217
Ng KL, Li JD, Cheng MY, Leslie FM, Lee AG, Zhou QY (2005) Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science 308:1923–1927
Nishikawa T, Mataga N, Takashima M, Toru M (1983) Behavioral sensitization and relative hyperresponsiveness of striatal and limbic dopaminergic neurons after repeated methamphetamine treatment. Eur J Pharmacol 88:195–203
Nishiyama T, Ikeda M, Iwata N, Suzuki T, Kitajima T, Yamanouchi Y et al (2005) Haplotype association between GABAA receptor gamma2 subunit gene (GABRG2) and methamphetamine use disorder. Pharmacogenomics J 5:89–95
NSDUH Substance Abuse and Mental Health Services Administration (2009) Results from the 2008 National Survey on Drug Use and Health: National Findings (Office of Applied Studies, NSDUH Series H-36, HHS Publication No. SMA 09–4434) Rockville, MD, pp 242–243
Ohgake S, Hashimoto K, Shimizu E, Koizumi H, Okamura N, Koike K et al (2005) Functional polymorphism of the NQO2 gene is associated with methamphetamine psychosis. Addict Biol 10:145–148
Okahisa Y, Ujike H, Kotaka T, Morita Y, Kodama M, Inada T et al (2009) Association between neuropeptide Y gene and its receptor Y1 gene and methamphetamine dependence. Psychiatry Clin Neurosci 63:417–422
Okochi T, Kishi T, Ikeda M, Kitajima T, Kinoshita Y, Kawashima K et al (2009) Genetic association analysis of NRG1 with methamphetamine-induced psychosis in a Japanese population. Prog Neuropsychopharmacol Biol Psychiatry 33:903–905
Okuyama S, Chaki S, Kawashima N, Suzuki Y, Ogawa S, Kumagai T et al (1997) The atypical antipsychotic profile of NRA0045, a novel dopamine D4 and 5-hydroxytryptamine2A receptor antagonist, in rats. Br J Pharmacol 121:515–525
Pulver AE, Lasseter VK, Kasch L, Wolyniec P, Nestadt G, Blouin JL et al (1995) Schizophrenia: a genome scan targets chromosomes 3p and 8p as potential sites of susceptibility genes. Am J Med Genet 60:252–260
Rawson RA, Gonzales R, Brethen P (2002) Treatment of methamphetamine use disorder: an update. J Subst Abuse Treat 23:145–150
Reichel CM, Schwendt M, McGinty J, Olive MF, See RE (2011) Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology [advance online publication]
Robinson TE, Becker JB (1986) Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res 396:157–198
Ross J, Berrettini W, Coryell W, Gershon ES, Badner JA, Kelsoe JR et al (2008) Genome-wide parametric linkage analyses of 644 bipolar pedigrees suggest susceptibility loci at chromosomes 16 and 20. Psychiatr Genet 18:191–198
Rothman RB, Baumann MH (2003) Monoamine transporters and psychostimulant drugs. Eur J Pharmacol 479:23–40
Rounsaville BJ, Anton SF, Carroll K, Budde D, Prusoff BA, Gawin F (1991) Psychiatric diagnoses of treatment-seeking cocaine abusers. Arch Gen Psychiatry 48:43–51
Salo R, Nordahl TE, Leamon MH, Natsuaki Y, Moore CD, Waters C et al (2008) Preliminary evidence of behavioral predictors of recurrent drug-induced psychosis in methamphetamine abuse. Psychiatry Res 157:273–277
SAMHSA [Substance Abuse and Mental Health Services Administration], Office of Applied Studies (2009) Treatment Episode Data Set (TEDS) 1998–2008, National Admissions to Substance Abuse Treatment Services, Drugs and Alcohol Services Information System Series: S-50, HHS Publication No. (SMA) 09–4471, Rockville, MD, 52
Sartorius LJ, Weinberger DR, Hyde TM, Harrison PJ, Kleinman JE, Lipska BK (2008) Expression of a GRM3 splice variant is increased in the dorsolateral prefrontal cortex of individuals carrying a schizophrenia risk SNP. Neuropsychopharmacology 33:2626–2634
Sato M (1992) A lasting vulnerability to psychosis in patients with previous methamphetamine psychosis. Ann N Y Acad Sci 654:160–170
Schumacher J, Jamra RA, Freudenberg J, Becker T, Ohlraun S, Otte AC et al (2004) Examination of G72 and D-amino-acid oxidase as genetic risk factors for schizophrenia and bipolar affective disorder. Mol Psychiatry 9:203–207
Segal DS, Mandell AJ (1974) Long-term administration of d-amphetamine: progressive augmentation of motor activity and stereotypy. Pharmacol Biochem Behav 2:249–255
Segal DS, Geyer MA, Schuckit MA (1981) Stimulant-induced psychosis: an evaluation of animal methods. Essays Neurochem Neuropharmacol 5:95–129
Segal DS, Kuczenski R (1997) Repeated binge exposures to amphetamine and methamphetamine: behavioral and neurochemical characterization. J Pharmacol Exp Ther 282:561–573
Segal DS, Kuczenski R (1999) Escalating dose-binge treatment with methylphenidate: role of serotonin in the emergent behavioral profile. J Pharmacol Exp Ther 291:19–30
Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H et al (2001) Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry 158:1206–1214
Sekine Y, Minabe Y, Ouchi Y, Takei N, Iyo M, Nakamura K et al (2003) Association of dopamine transporter loss in the orbitofrontal and dorsolateral prefrontal cortices with methamphetamine-related psychiatric symptoms. Am J Psychiatry 160:1699–1701
Shinba T, Yamamoto K, Cao GM, Mugishima G, Andow Y, Hoshino T (1996) Effects of acute methamphetamine administration on spacing in paired rats: investigation with an automated video-analysis method. Prog Neuropsychopharmacol Biol Psychiatry 20:1037–1049
Shoptaw S, Peck J, Reback FJ, Rotheram-Fuller E (2003) Psychiatric and susbstance dependence comorbidities, sexually transmitted diseases, and risk behaviors among methamphetamine-dependenct gay and bisexual men seeking outpatient drug abuse treatment. J Psychoactive Drugs 35(Suppl1):161–168
Srisurapanont M, Ali R, Marsden J, Sunga A, Wada K, Monteiro M (2003) Psychotic symptoms in methamphetamine psychotic in-patients. Int J Neuropsychopharmacol 6:347–352
Stone DK, Reynolds AD, Mosley RL, Gendelman HE (2009) Innate and adaptive immunity for the pathobiology of Parkinson’s disease. Antioxid Redox Signal 11:2151–2166
Straub RE, Jiang Y, Maclean CJ, Ma Y, Webb BT, Myakishev MV et al (2002) Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am J Hum Genet 71:337–348
Suzuki A, Nakamura K, Sekine Y, Minabe Y, Takei N, Suzuki K et al (2006) An association study between catechol-O-methyl transferase gene polymorphism and methamphetamine psychotic disorder. Psychiatr Genet 16:133–138
Takigawa M, Maeda H, Ueyama K, Tominaga H, Matsumoto K (1993) A dual approach to self-stimulation and locomotor trace affected by chronic methamphetamine treatment for an animal model of schizophrenia. Can J Physiol Pharmacol 71:321–325
Talbot K, Eidem WL, Tinsley CL, Benson MA, Thompson EW, Smith RJ et al (2004) Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. J Clin Invest 113:1353–1363
Thomas DM, Francescutti-Verbeem DM, Liu X, Kuhn DM (2004a) Identification of differentially regulated transcripts in mouse striatum following methamphetamine treatment–an oligonucleotide microarray approach. J Neurochem 88:380–393
Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM (2004b) Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharmacol Exp Ther 311:1–7
Thomas DM, Francescutti-Verbeem DM, Kuhn DM (2008a) The newly synthesized pool of dopamine determines the severity of methamphetamine-induced neurotoxicity. J Neurochem 105:605–616
Thomas DM, Francescutti-Verbeem DM, Kuhn DM (2008b) Methamphetamine-induced neurotoxicity and microglial activation are not mediated by fractalkine receptor signaling. J Neurochem 106:696–705
Tsunoka T, Kishi T, Kitajima T, Okochi T, Okumura T, Yamanouchi Y et al (2010) Association analysis of GRM2 and HTR2A with methamphetamine-induced psychosis and schizophrenia in the Japanese population. Prog Neuropsychopharmacol Biol Psychiatry 34:639–644
Ujike H (2002) Stimulant-induced psychosis and schizophrenia: the role of sensitization. Curr Psychiatry Rep 4:177–184
Ujike H, Sato M (2004) Clinical features of sensitization to methamphetamine observed in patients with methamphetamine dependence and psychosis. Ann NY Acad Sci 1025:279–287
Ujike H, Ogawa N, Otsuki S (1988) Effects of acute and long-term treatment with methamphetamine on substance P concentration and receptor numbers in the rat brain. Brain Res 453:136–142
Ujike H, Onoue T, Akiyama K, Hamamura T, Otsuki S (1989) Effect of selective D-1 and D-2 dopamine antagonists on development of methamphetamine-induced behavioral sensitization. Psychopharmacology 98:89–92
Ujike H, Kanzaki A, Okumura K, Akiyama K, Otsuki S (1992) Sigma (sigma) antagonist BMY 14802 prevents methamphetamine-induced sensitization. Life Sci 50:PL129–PL134
Ujike H, Harano M, Inada T, Yamada M, Komiyama T, Sekine Y et al (2003) Nine- or fewer repeat alleles in VNTR polymorphism of the dopamine transporter gene is a strong risk factor for prolonged methamphetamine psychosis. Pharmacogenomics J 3:242–247
Ujike H, Sakai A, Nakata K, Tanaka Y, Kodaka T, Okahisa Y et al (2006) Association study of the dihydropyrimidinase-related protein 2 gene and methamphetamine psychosis. Ann N Y Acad Sci 1074:90–96
Ujike H, Katsu T, Okahisa Y, Takaki M, Kodama M, Inada T et al (2009) Genetic variants of D2 but not D3 or D4 dopamine receptor gene are associated with rapid onset and poor prognosis of methamphetamine psychosis. Prog Neuropsychopharmacol Biol Psychiatry 33:625–629
UNODC United Nations Office on Drugs and Crime, World Drug Report (2009) http://www.unodc.org/documents/scientific/ATS/Global-ATS-Assessment-2009-Web.pdf, 280
Utena H (1961) A special type of model psychosis: a chronic methamphetamine intoxication in man and animal. Brain Nerve 13:687–692
Utena H (1966) Behavioral aberrations in methamphetamine-intoxicated animals and chemical correlates in the brain. Prog Brain Res 21B:192–207
van Os J, Bak M, Hanssen M, Bijl RV, De Graaf R, Verdoux H (2002) Cannabis use and psychosis: a longitudinal population based study. Am J Epidemiol 156:319–327
Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M et al (2001a) Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry 158:2015–2021
Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M et al (2001b) Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci 21:9414–9418
Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D et al (2001c) Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry 158:377–382
Volz TJ, Fleckenstein AE, Hanson GR (2007) Methamphetamine-induced alterations in monoamine transport: implications for neurotoxicity, neuroprotection and treatment. Addiction 102(Suppl 1):44–48
Wallace C, Galloway T, McKetin R, Kelly E, Leary J (2009) Methamphetamine use, dependence and treatment access in rural and regional North Coast of New South Wales, Australia. Drug Alcohol Rev 28:592–599
Wang HY, Liu T, Malbon CC (2006a) Structure-function analysis of Frizzleds. Cell Signal 18:934–941
Wang Y, Zhang J, Mori S, Nathans J (2006b) Axonal growth and guidance defects in Frizzled3 knock-out mice: a comparison of diffusion tensor magnetic resonance imaging, neurofilament staining, and genetically directed cell labeling. J Neurosci 26:355–364
Weickert CS, Miranda-Angulo AL, Wong J, Perlman WR, Ward SE, Radhakrishna V et al (2008) Variants in the estrogen receptor alpha gene and its mRNA contribute to risk for schizophrenia. Hum Mol Genet 17:2293–2309
Weisner C, Mertens J, Parthasarathy S, Moore C, Hunkeler EM, Hu T et al (2000) The outcome and cost of alcohol and drug treatment in an HMO: day hospital versus traditional outpatient regimens. Health Serv Res 35:791–812
Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM et al (1996) Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med 2:699–703
Xu W, Zhu JP, Angulo JA (2005) Induction of striatal pre- and postsynaptic damage by methamphetamine requires the dopamine receptors. Synapse 58:110–121
Yagi M (1963) Factors influencing the general activity in rats: 2. Effects of methamphetamine. Ann Anim Psychol 13:37–47
Yang J, Si T, Ling Y, Ruan Y, Han Y, Wang X et al (2003) Association study of the human FZD3 locus with schizophrenia. Biol Psychiatry 54:1298–1301
Yen CF, Chong MY (2006) Comorbid psychiatric disorders, sex, and methamphetamine use in adolescents: a case-control study. Compr Psychiatry 47:215–220
Yoo JH, Cho JH, Yu HS, Lee KW, Lee BH, Jeong SM et al (2006) Involvement of 5-HT receptors in the development and expression of methamphetamine-induced behavioral sensitization: 5-HT receptor channel and binding study. J Neurochem 99:976–988
Yoshikawa T, Lin SK, Mori N (2006) Association analysis of SOD2 variants with methamphetamine psychosis in Japanese and Taiwanese populations. Hum Genet 120:243–252
Yue W, Kang G, Zhang Y, Qu M, Tang F, Han Y et al (2007) Association of DAOA polymorphisms with schizophrenia and clinical symptoms or therapeutic effects. Neurosci Lett 416:96–100
Yui K, Goto K, Ikemoto S, Ishiguro T, Kamada Y (1999) Increased sensitivity to stress and episode recurrence in spontaneous recurrence of methamphetamine psychosis. Psychopharmacology 145:267–272
Yui K, Ikemoto S, Ishiguro T, Goto K (2000) Studies of amphetamine or methamphetamine psychosis in Japan: relation of methamphetamine psychosis to schizophrenia. Ann N Y Acad Sci 914:1–12
Zhang Y, Yu X, Yuan Y, Ling Y, Ruan Y, Si T et al (2004) Positive association of the human frizzled 3 (FZD3) gene haplotype with schizophrenia in Chinese Han population. Am J Med Genet B Neuropsychiatr Genet 129B:16–19
Zweben JE, Cohen JB, Christian D, Galloway GP, Salinardi M, Parent D et al (2004) Psychiatric symptoms in methamphetamine users. Am J Addict 13:181–190
Acknowledgements
This work was support in part by the National Institutes of Health grant 1P01 DA028555-01
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Grant, K.M., LeVan, T.D., Wells, S.M. et al. Methamphetamine-Associated Psychosis. J Neuroimmune Pharmacol 7, 113–139 (2012). https://doi.org/10.1007/s11481-011-9288-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-011-9288-1