Abstract
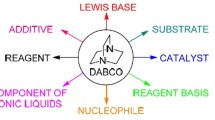
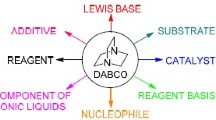
The organic reactions catalyzed by 1, 4-diazabicyclo [2.2.2] octane (DABCO) are reviewed. Most of the reactions start conveniently from available substrate and proceed under mild conditions. The reactions are environmentally friendly and the catalyst can be recycled in some cases. The perspectives on DABCO-catalyzed reactions are pointed out.
Similar content being viewed by others
References
Baylis A B, Hillman M E D. Offenlegungsschrift 2155113, 1972; U.S. Patent 3,743,669; Chem Abstr, 1972, 77, 34174q
Rafel S, Leahy J W. An unexpected rate acceleration-practical improvements in the Baylis-Hillman reaction. J Org Chem, 1997, 62(5): 1521–1552
Kaye P T, Nocanda X W. Application of Baylis-Hillman methodology in a chemoselective synthesis of 3-acyl-2H-1-chromenes. J Chem Soc, Perkin Trans, 2000, 1: 1331–1332
Lee K Y, Kim J M, Kim J N. Facile synthesis of 3-alkylidene-3H-isobenzofuranones from the Baylis-Hillman reaction of 2-carboxybenzaldehyde. Synlett, 2003, (3): 357–360
Faltin C, Fleming E M, Connon S J. Acrylamide in the Baylis-Hillman reaction: expanded reaction scope and the unexpected superiority of DABCO over more basic tertiary Amine catalysts. J Org Chem, 2004, 69(19): 6496–6499
Kaye P T, Nocanda X W. A convenient general synthesis of 3-substituted 2H-chromene derivatives. J Chem Soc, Perkin Trans, 2002, 1: 1318–1323
Coelho F, Almeida W P, Veronese D, Mateus C R, Silva Lopes E C, Rossi R C, Silveira G P C, Pavam C H. Ultrasound in Baylis-Hillman reactions with aliphatic and aromatic aldehydes: scope and limitations. Tetrahedron, 2002, 58(37): 7437–7447
De Souza R O M A, Meireles B A, Aguiar L C S, Vasconcellos M L A A. Hexamethylenetetramine as a cheap and convenient alternative catalyst in the Baylis-Hillman reaction: synthesis of aromatic compounds with anti-malarial activity. Synthesis, 2004, (10): 1595–1600
Areces P, Carrasco E, Mancha A, Plumet J. Tandem B-elimination-morita-Baylis-Hillman reaction in A,B-unsaturated sugar aldehydes. Synthesis, 2006, (6): 946–948
Shi M, Li C Q, Jiang J K. Reexamination of the traditional Baylis-Hillman reaction. Tetrahedron, 2003, 59(8): 1181–1189
Raheem I T, Jacobsen E N. Highly enantioselective aza-Baylis-Hillman reactions catalyzed by chiral thiourea derivatives. Adv Synth Catal, 2005, 347: 1701–1708
Drewes S E, Emslie N D, Karodia N, Khan A A. Facile diastereoselective synthesis of 2,6-dialkyl-5-methylene-1,3-dioxan-4-ones via A-activated vinyl esters. Chem Ber, 1990, 123: 1447–1448
Matsuya Y, Hayashi K, Nemoto H. A novel modified Baylis-Hillman reaction of propiolate. J Am Chem Soc, 2003, 125(3): 646–647
Shi M, Zhao G L. Aza-Baylis-Hillman reactions of diisopropyl azodicarboxylate or diethyl azodicarboxylate with acrylates and acrylonitrile. Tetrahedron, 2004, 60(9): 2083–2089
Turki T, Villiéras J, Amri H. An efficient synthesis of alkyl A-(hydroxymethyl)acrylates induced by DABCO in an aqueous medium. Tetrahedron letters, 2005, 46(17): 3071–3072
Saxena R, Patra A, Batra S. An alternate route to substituted 1, 4-pentanedienes through acetates of Baylis-Hillman adducts in aqueous medium. Synlett, 2003, (10): 1439–1442
Yu C Z, Lin B, Hu L. Efficient Baylis-Hillman reaction using stoichiometric base catalyst and an aqueous medium. J Org Chem, 2001, 66(16): 5413–5418
Papageorgiou C D, Ley S V, Gaunt M J. Organic-catalyst-mediated cyclopropanation reaction. Angew Chem Int Ed, 2003, 42(7): 828–831
Bremeyer N, Smith S C, Ley S V, Gaunt M J. An intramolecular organocatalytic cyclopropanation reaction. Angew Chem, 2004, 116: 2735–2738
Papageorgiou C D, Cubillo de Dios M A, Ley S V, Gaunt M J. Enantioselective organocatalytic cyclopropanation via ammonium ylides. Angew Chem Int Ed, 2004, 43: 4641–4644
Kimachi T, Kinoshita H, Kusaka K, Takeuchi Y, Aoe M, Juichi M. The highly trans-selective darzens reaction via ammonium ylides. Synlett, 2005, (5): 842–844
Zhao G L, Shi M. Aza-Baylis-Hillman reactions of N-tosylated aldimines with activated allenes and alkynes in the presence of various Lewis Base promoters. J Org Chem, 2005, 70(24): 9975–9984
Yang Z J, Fan M J, Mu R Z, Liu W M, Liang Y M. A facile synthesis of highly functionalized dihydrofurans based on 1, 4-diazabicyclo[2.2.2]octane(DABCO) catalyzed reaction of halides with enones. Tetrahedron, 2005, 61(38): 9140–9146
Shi L, Han Y, Yang Z J, Liu W M, Liang Y M. Stereoselective synthesis of trans-B-substituted X-ferrocenyl-X-butyrolactones via ammonium ylides. Synthesis, 2005, (17): 2851–2856
Fan M J, Yan Z Y, Liu W M, Liang Y M. DABCO-Catalyzed reaction of A-halo carbonyl compounds with dimethyl acetylenedicarboxylate: a novel method for the preparation of polysubstituted furans and highly functionalized 2H-pyrans. J Org Chem, 2005, 70(20): 8204–8207
Fan M J, Guo L N, Liu X Y, Liu W M, Liang Y M. A mild, convenient and efficient single-step method for the synthesis of polysubstituted furans via ammonium ylide routes. Synthesis, 2005, (3): 391–396
Fan M J, Li G Q, Liang Y M. DABCO catalyzed reaction of various nucleophiles with activated alkynes leading to the formation of alkenoic acid esters, 1,4-dioxane, morpholine, and piperazinone derivatives. Tetrahedron, 2006, 62(29): 6782–6791
Fan M J, Li G Q, Li L H, Yang S D, Liang Y M. DABCO-Catalyzed reaction of phenols or 1,2-diphenols with activated alkynes leading to the formation of alkenoic acid esters or 1,3-dioxole derivatives. Synthesis, 2006, (14): 2286–2292
Shi Y L, Shi M. DABCO-Catalyzed reaction of allenic esters and ketones with salicyl N-tosylimines: synthesis of highly functionalized chromenes. Org Lett, 2005, 7(14): 3057–3060
Shi Y L, Shi M. Synthesis of substituted chromenes through the DABCO-Catalyzed reaction of but-3-yn-2-one and methyl propiolate with salicyl N-tosylimines. Chem Eur J, 2006, 12: 3374–3378
Li J H, Liu W J. DABCO as an inexpensive and highly efficient ligand for palladium-catalyzed Suzuki-Miyaura cross-coupling reaction. Org Lett, 2004, 6(16): 2809–2811
Li J H, Hu X C, Liang Y, Xie Y X. PEG-400 promoted Pd (OAc)2/DABCO-catalyzed cross-coupling reactions in aqueous media. Tetrahedron, 2006, 62(1): 31–38
Li J H, Liu W J, Xie Y X. Recyclable and Reusable Pd(OAc)2/DABCO/PEG-400 system for Suzuki-Miyaura cross-coupling reaction. J Org Chem, 2005, 70(14): 5409–5412
Li J H, Wang D P. CuI/DABCO-Catalyzed cross-coupling reactions of aryl halides with arylboronic acids. Eur J Org Chem, 2006, 2063–2066
Li J H, Liang Y, Wang D P, Liu W J, Xie Y X, Yin D L. Efficient Stille cross-coupling reaction catalyzed by the Pd(OAc)2/DABCO catalytic system. J Org Chem, 2005, 70(7): 2832–2834
Li J H, Deng C L, Liu W J, Xie Y X. Pd(OAc)2/DABCO as an inexpensive and efficient catalytic system for Hiyama cross-coupling reactions of aryl halides with aryltrimethoxysilanes. Synthesis, 2005, (18): 3039–3044
Li J H, Zhu Q M, Xie Y X. Pd(OAc)2/DABCO-catalyzed Suzuki-Miyaura cross-coupling reaction in DMF. Tetrahedron, 2006, 62(47): 10888–10895
Xie Y X, Li J H, Yin D L. Amines as the ligands for Palladium-catalyzed coupling reaction. Chinese Journal of Organic Chemistry, 2006, 26(8): 1155–1163
Li J H, Zhang X D, Xie Y X. Efficient and copper-free Pd(OAc)2/DABCO-catalyzed Sonogashira cross-coupling reaction. Synthesis, 2005, (5): 804–808
Li J H, Wang D P, Xie Y X. Pd(OAc)2/DABCO as a highly active catalytic system for the Heck reaction. Synthesis, 2005, (13): 2193–2197
Li J H, Wang D P, Xie Y X. CuI/DABCO as a highly active catalytic system for the Heck-type reaction. Tetrahedron letters, 2005, 46(30): 4941–4944
Shieh W C, Dell S, Bach A, Repič O, Blacklock T J. Dual nucleophilic catalysis with DABCO for the N-methylation of Indoles. J Org Chem, 2003, 68(5): 1954–1957
Shieh W C, Lozanov M, Loo M, Repic O, Blacklock T J. DABCO-and DBU-accelerated green chemistry for N-, O-, and S-benzylation with dibenzyl carbonate. Tetrahedron letters, 2003, 44(24): 4563–4565
Lesch B, Toräng J, Vanderheiden S, Bräse S. Base-catalyzed condensation of 2-hydroxybenzaldehydes with A,B-unsaturated aldehydes-scope and limitations. Adv Synth Catal, 2005, 347: 555–562
Sonye J P, Koide K. On the mechanism of DABCO-catalyzed isomerization of X-hydroxy-A,B-alkynoates to X-oxo-A, B-(E)-alkenoates. Org Lett, 2006, 8(2): 199–202
Sonye J P, Koide K. Base-catalyzed stereoselective isomerization of electron-deficient propargylic alcohols to E-enones. J Org Chem, 2006, 71(16): 6254–6257
Fioravanti S, Colantoni D, Pellacani L, Tardella P A. Aziridines versus vinyl carbamates from the direct amination of electron-withdrawing group-substituted trifluoromethyl enoates. J Org Chem, 2005, 70(8): 3296–3298
Uozumi Y, Arii T, Watanabe T. Double carbonylation of aryl iodides with primary amines under atmospheric pressure conditions using the Pd/PPh3/DABCO/THF system. J Org Chem, 2001, 66(15): 5272–5247
Shi Y J, Humphrey G, Maligres P E, Reamer R A, Michael Williams J. Highly regioselective DABCO-catalyzed nucleophilic aromatic substitution reaction of methyl 2,6-dichloronicotinate with phenols. Adv Synth Catal, 2006, 348: 309–312
Author information
Authors and Affiliations
Corresponding author
Additional information
__________
Translated from Chemistry online, 2007, 70(10): 759–765 [译自: 化学通报]
About this article
Cite this article
Yang, H., Tian, R. & Li, Y. Organic reactions catalyzed by 1, 4-diazabicyclo [2.2.2] octane (DABCO). Front. Chem. China 3, 279–287 (2008). https://doi.org/10.1007/s11458-008-0049-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11458-008-0049-5