Abstract
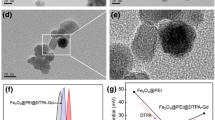
Multifunctional nanoparticles combining diagnostic and therapeutic agents into a single platform make cancer theranostics possible and have attracted wide interests in the field. In this study, a multifunctional nanocomposite based on dextran and superparamagnetic iron oxide nanoparticles (SPIO) was prepared for drug delivery and magnetic resonance imaging (MRI). Amphiphilic dextran was synthesized by grafting stearyl acid onto the carbohydrate backbone, and micelle was formed by the resulted amphiphilic dextran with low critical micelle concentration at 1.8 mg L−1. Doxorubicin (DOX) and a cluster of the manganese-doped iron oxide nanoparticles (Mn-SPIO) nanocrystals were then coencapsulated successfully inside the core of dextran micelles, resulting in nanocomposites with diameter at about 100 nm. Cell culture experiments demonstrated the potential of these Mn-SPIO/DOX nanocomposites as an effective multifunctional nanoplatform for the delivery of anticancer drug DOX with a loading content (DLC) of 16 %. Confocal laser scanning microscopy reveals that the Mn-SPIO/DOX had excellent internalization ability against MCF-7/Adr cells after 2-h labeling compared with free DOX·HCl. Under a 3.0-T MRI scanner, Mn-SPIO/DOX nanocomposite-labeled cells in gelatin phantom show much darker images than the control. Their transverse relaxation (T 2) rate is also significantly higher than that of the control cells (33.9 versus 2.3 s−1). Our result offers an effective strategy to treat MCF-7/Adr at optimized low dosages with imaging capability.
摘要
多功能纳米颗粒能同时结合诊断和治疗试剂,有望实现肿瘤诊疗的一体化。本文制备了基于葡聚糖和超顺磁氧化铁纳米颗粒的多功能纳米复合物DOX/Mn-SPIO,用于药物传递和磁共振成像(MRI)。通过接枝硬脂酸到糖单元骨架上,获得临界胶束浓度为1.8 mg L−1的两亲性葡聚糖。经自组装的葡聚糖胶束可在疏水核负载抗肿瘤药物阿霉素和超顺磁氧化锰铁纳米晶体,形成粒径约100 nm的纳米复合物。体外细胞实验表明,该DOX/Mn-SPIO纳米复合物能作为有效传递抗肿瘤药物阿霉素的多功能纳米载体。相比游离的阿霉素药物,激光共聚焦显微镜结果表明该复合物在标记2 h时可高效进入人乳腺癌耐药细胞MCF-7/Adr。使用临床3 T磁共振扫描仪成像,该复合物标记的细胞MRI图像与对照组显示出明显对比度。其T 2弛豫率达33.9 s−1,显著高于对照组的2.3 s−1。研究表明该葡聚糖纳米胶束复合物作为诊疗一体化载体能在低剂量治疗MCF-7/Adr细胞的同时进行MRI成像,为MCF-7/Adr肿瘤细胞的诊疗提供了一种有效方法。








Similar content being viewed by others
References
Yoon TJ, Yu KN, Kim E et al (2006) Specific targeting, cell sorting, and bioimaging with smart magnetic silica core–shell nanomaterials. Small 2:209–215
Chu M, Song X, Cheng D et al (2006) Preparation of quantum dot-coated magnetic polystyrene nanospheres for cancer cell labelling and separation. Nanotechnology 17:3268
Sathe TR, Agrawal A, Nie S (2006) Mesoporous silica beads embedded with semiconductor quantum dots and iron oxide nanocrystals: dual-function microcarriers for optical encoding and magnetic separation. Anal Chem 78:5627–5632
Mulder WJM, Koole R, Brandwijk RJ et al (2005) Quantum dots with a paramagnetic coating as a bimodal molecular imaging probe. Nano Lett 6:1–6
Quarta A, Di Corato R, Manna L et al (2008) Multifunctional nanostructures based on inorganic nanoparticles and oligothiophenes and their exploitation for cellular studies. J Am Chem Soc 130:10545–10555
Wang D, Su H, Liu Y et al (2012) Near-infrared fluorescent amphiphilic polycation wrapped magnetite nanoparticles as multimodality probes. Chin Sci Bull 57:4012–4018
Yang F, Li Y, Chen Z et al (2009) The preparation and application of microbubble contrast agent combining ultrasound imaging and magnetic resonance imaging. Chin Sci Bull 54:2934–2939
Wang X, Zhang J, Yang X et al (2014) In vivo assessment of hepatotoxicity, nephrotoxicity and biodistribution using 3-aminopropyltriethoxysilane-coated magnetic nanoparticles (APTS-MNPs) in ICR mice. Chin Sci Bull 59:1800–1808
Torchilin VP (2006) Multifunctional nanocarriers. Adv Drug Deliv Rev 58:1532–1555
Sun C, Lee JSH, Zhang M (2008) Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev 60:1252–1265
Luo JP, Qu SX, Liu JT et al (2013) Rapid detection of Cyfra 21-1 by optical-biosensing based on chemiluminescence immunoassay using bio-functionalized magnetic nanocomposites. Chin Sci Bull 58:2567–2569
Knipe JM, Peppas NA (2014) Multi-responsive hydrogels for drug delivery and tissue engineering applications. Regener Biomater 1:57–65
Cai TT, Lei Q, Yang B et al (2014) Utilization of h-bond interaction of nucleobase uralic with antitumor methotrexate to design drug carrier with ultrahigh loading efficiency and ph-responsive drug release. Regener Biomater 1:27–35
Nasongkla N, Bey E, Ren J et al (2006) Multifunctional polymeric micelles as cancer-targeted, mri-ultrasensitive drug delivery systems. Nano Lett 6:2427–2430
Guo R, Zhang L, Qian H et al (2010) Multifunctional nanocarriers for cell imaging, drug delivery, and near-IR photothermal therapy. Langmuir 26:5428–5434
Bhaskar S, Tian F, Stoeger T et al (2010) Multifunctional nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: perspectives on tracking and neuroimaging. Part Fibre Toxicol 7:3
Zhou J, Huang L, Wang W et al (2009) Prostate cancer targeted MRI nanoprobe based on superparamagnetic iron oxide and copolymer of poly (ethylene glycol) and polyethyleneimin. Chin Sci Bull 54:3137–3146
Xie J, Liu G, Eden HS et al (2011) Surface-engineered magnetic nanoparticle platforms for cancer imaging and therapy. Accounts Chem Res 44:883–892
Weissleder R, Mahmood U (2001) Molecular imaging. Radiology 219:316–333
Herschman HR (2003) Molecular imaging: looking at problems, seeing solutions. Science 302:605–608
Yan G, Zhuo R (2001) Research progress of magnetic resonance imaging contrast agents. Chin Sci Bull 46:1233–1237
Wang YX, Hussain S, Krestin G (2001) Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur Radiol 11:2319–2331
Thorek D, Chen A, Czupryna J et al (2006) Superparamagnetic iron oxide nanoparticle probes for molecular imaging. Ann Biomed Eng 34:23–38
Feng L, Song Y, Wan M et al (2001) Research progress of magnetic iron oxide nanoparticles. Chin Sci Bull 46:1321–1325
Jin RR, Lin BB, Li DY et al (2014) Superparamagnetic iron oxide nanoparticles for MR imaging and therapy: design considerations and clinical applications. Curr Opin Pharmacol 18:18–27
Tromsdorf UI, Bigall NC, Kaul MG et al (2007) Size and surface effects on the MRI relaxivity of manganese ferrite nanoparticle contrast agents. Nano Lett 7:2422–2427
Lee JH, Huh YM, Jun YW et al (2007) Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat Med 13:95–99
Hong G, Yuan R, Liang B et al (2008) Folate-functionalized polymeric micelle as hepatic carcinoma-targeted, MRI-ultrasensitive delivery system of antitumor drugs. Biomed Microdevices 10:693–700
Yang X, Grailer JJ, Rowland IJ et al (2010) Multifunctional SPIO/DOX-loaded wormlike polymer vesicles for cancer therapy and MR imaging. Biomaterials 31:9065–9073
Lu J, Ma SL, Sun JY et al (2009) Manganese ferrite nanoparticle micellar nanocomposites as MRI contrast agent for liver imaging. Biomaterials 30:2919–2928
Kakizawa Y, Kataoka K (2002) Block copolymer micelles for delivery of gene and related compounds. Adv Drug Deliv Rev 54:203–222
Savic R, Eisenberg A, Maysinger D (2006) Block copolymer micelles as delivery vehicles of hydrophobic drugs: micelle-cell interactions. J Drug Target 14:343–355
Nasongkla N, Shuai X, Ai H et al (2004) cRGD-functionalized polymer micelles for targeted doxorubicin delivery. Angew Chem Int Ed 43:6323–6327
Shuai XT, Ai H, Nasongkla N et al (2004) Micellar carriers based on block copolymers of poly(ε-caprolactone) and poly(ethylene glycol) for doxorubicin delivery. J Control Release 98:415–426
Cao YW, Gao M, Chen C et al (2015) Triggered-release polymeric conjugate micelles for on-demand intracellular drug delivery. Nanotechnology 26:115101–115110
Croy SR, Kwon GS (2006) Polymeric micelles for drug delivery. Curr Pharm Design 12:4669–4684
Chen YQ, Cao J, Zhu HY et al (2013) Synthesis and evaluation of methionine and folate co-decorated chitosan self-assembly polymeric micelles as a potential hydrophobic drug-delivery system. Chin Sci Bull 58:2379–2386
Ai H, Flask C, Weinberg B et al (2005) Magnetite-loaded polymeric micelles as ultrasensitive magnetic-resonance probes. Adv Mater 17:1949–1952
Liu G, Wang ZY, Lu J et al (2011) Low molecular weight alkyl-polycation wrapped magnetite nanoparticle clusters as MRI probes for stem cell labeling and in vivo imaging. Biomaterials 32:528–537
de Jonge E, Levi M (2001) Effects of different plasma substitutes on blood coagulation: a comparative review. Crit Care Med 29:1261–1267
Messmore HL, Jeske WP, Wehrmacher WH et al (2003) Benefit-risk assessment of treatments for heparin-induced thrombocytopenia. Drug Saf 26:625–641
Ikeda Y, Charef S, Ouidja MO et al (2011) Synthesis and biological activities of a library of glycosaminoglycans mimetic oligosaccharides. Biomaterials 32:769–776
Zhu Y, Tong WJ, Gao CY et al (2008) Fabrication of bovine serum albumin microcapsules by desolvation and destroyable cross-linking. J Mater Chem 18:1153–1158
McArthur SL, McLean KM, Kingshott P et al (2000) Effect of polysaccharide structure on protein adsorption. Colloids Surf B 17:37–48
Sun SH, Zeng H, Robinson DB et al (2004) Monodisperse MFe2O4 (M = Fe Co, Mn) nanoparticles. J Am Chem Soc 126:273–279
Su HY, Liu YH, Wang D et al (2013) Amphiphilic starlike dextran wrapped superparamagnetic iron oxide nanoparticle clsuters as effective magnetic resonance imaging probes. Biomaterials 34:1193–1203
Wang QY, Su HY, Xia CC et al (2009) Amphiphilic dextran/magnetite nanocomposites as magnetic resonance imaging probes. Chin Sci Bull 54:2925–2933
Karukstis KK, Thompson EHZ, Whiles JA et al (1998) Deciphering the fluorescence signature of daunomycin and doxorubicin. Biophys Chem 73:249–263
Kircher MF, Allport JR, Graves EE et al (2003) In vivo high resolution three–dimensional imaging of antigen-specific cytotoxic T-lymphocyte trafficking to tumors. Cancer Res 63:6838–6846
Song HT, Choi JS, Huh YM et al (2005) Surface modulation of magnetic nanocrystals in the development of highly efficient magnetic resonance probes for intracellular labeling. J Am Chem Soc 127:9992–9993
Acknowledgments
This work was supported by the National Basic Research Program of China (2013CB933903), the National Key Technology Research and Development Program (2012BAI23B08), the National Natural Science Foundation of China (51173117), and the Scientific Research Start-up Fund of Kunming University of Science and Technology (KKSY201305089).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Bingbing Lin and Hongying Su contributed equally to this work.
About this article
Cite this article
Lin, B., Su, H., Jin, R. et al. Multifunctional dextran micelles as drug delivery carriers and magnetic resonance imaging probes. Sci. Bull. 60, 1272–1280 (2015). https://doi.org/10.1007/s11434-015-0840-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-015-0840-x