Abstract
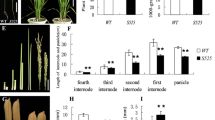
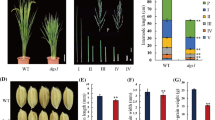
Brassinosteroids (BRs) are important hormones that regulate plant development and physiology. Substantial progresses have been made in BR-related studies, and especially an increasing number of new genes involved in BR biosynthesis have been identified. Here, we characterize a BR-related rice mutant, small grain 4 (sg4), obtained from callus culture of japonica cultivar Nipponbare. This mutant showed multiple phenotypes such as dark green, rugose erect leaves and small round grains. It was higher than the wild type, different from the majority of BR- and gibberellin-related mutants. Genetic analysis showed that the mutant phenotypes are controlled by a single recessive locus. The gene was fine-mapped to a 90.7-kb interval with 1,100 F2 recessive individuals by means of map-based cloning. Totally 11 open reading frames were found in this interval, only one of which was detected with an 8-bp insertion in the 5′UTR region by sequencing. Functional complementation test revealed that a DWARF11 allele, LOC_Os04g39430, is answer for the mutant phenotype of sg4. Tissue-specific response to BR and decreased expression levels of BR biosynthetic genes suggest that sg4 is a weak BR-deficient mutant. These results are beneficial to understanding the physiological action of sg4 in a more comprehensive way.






Similar content being viewed by others
References
Sasaki A, Itoh H, Gomi K et al (2003) Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science 299:1896–1898
Gomi K, Sasaki A, Itoh H et al (2004) GID2, an F-box subunit of the SCF E3 complex, specifically interacts with phosphorylated SLR1 protein and regulates the gibberellin-dependent degradation of SLR1 in rice. Plant J 37:626–634
Hong Z, Ueguchi-Tanaka M, Shimizu-Sato S et al (2002) Loss-of-function of a rice brassinosteroid biosynthetic enzyme, C-6 oxidase, prevents the organized arrangement and polar elongation of cells in the leaves and stem. Plant J 32:495–508
Hong Z, Ueguchi-Tanaka M, Umemura K et al (2003) A rice brassinosteroid-deficient mutant, ebisu dwarf (d2), is caused by a loss of function of a new member of cytochrome p450. Plant Cell 15:2900–2910
Hu X, Qian Q, Xu T et al (2013) The U-box E3 ubiquitin ligase TUD1 functions with a heterotrimeric G alpha subunit to regulate brassinosteroid-mediated growth in rice. PLoS Genet 9:e1003391
Sakamoto T, Morinaka Y, Ohnishi T et al (2006) Erect leaves caused by brassinosteroid deficiency increase biomass production and grain yield in rice. Nat Biotechnol 24:105–109
Tong H, Jin Y, Liu W et al (2009) Dwarf and low-tillering, a new member of the GRAS family, plays positive roles in brassinosteroid signaling in rice. Plant J 58:803–816
Yamamuro C, Ihara Y, Wu X et al (2000) Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint. Plant Cell 12:1591–1605
Tanabe S, Ashikari M, Fujioka S et al (2005) A novel cytochrome p450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. Plant Cell 17:776–790
Clouse SD (2011) Brassinosteroid signal transduction: from receptor kinase activation to transcriptional networks regulating plant development. Plant Cell 23:1219–1230
Chung Y, Choe S (2013) The regulation of brassinosteroid biosynthesis in Arabidopsis. Crit Rev Plant Sci 32:396–410
Mori M, Nomura T, Ooka H et al (2002) Isolation and characterization of a rice dwarf mutant with a defect in brassinosteroid biosynthesis. Plant Physiol 130:1152–1161
Gendron JM, Wang Z-Y (2007) Multiple mechanisms modulate brassinosteroid signaling. Curr Opin Plant Biol 10:436–441
Kim T-W, Wang Z-Y (2010) Brassinosteroid signal transduction from receptor kinases to transcription factors. Annu Rev Plant Biol 61:681–704
Bai MY, Zhang LY, Gampala SS et al (2007) Functions of OsBZR1 and 14-3-3 proteins in brassinosteroid signaling in rice. Proc Natl Acad Sci USA 104:13839–13844
Huelsken J, Birchmeier W (2001) New aspects of Wnt signaling pathways in higher vertebrates. Curr Opin Genet Dev 11:547–553
Wang L, Xu YY, Ma QB et al (2006) Heterotrimeric G protein alpha subunit is involved in rice brassinosteroid response. Cell Res 16:916–922
Gao Y, Wang S, Asami T et al (2008) Loss-of-function mutations in the Arabidopsis heterotrimeric G-protein alpha subunit enhance the developmental defects of brassinosteroid signaling and biosynthesis mutants. Plant Cell Physiol 49:1013–1024
Ueguchi-Tanaka M, Fujisawa Y, Kobayashi M et al (2000) Rice dwarf mutant d1, which is defective in the α subunit of the heterotrimeric G protein, affects gibberellin signal transduction. Proc Natl Acad Sci USA 97:11638–11643
Tanaka A, Nakagawa H, Tomita C et al (2009) Brassinosteroid upregulated 1, encoding a helix-loop-helix protein, is a novel gene involved in brassinosteroid signaling and controls bending of the lamina joint in rice. Plant Physiol 151:669–680
Tong H, Liu L, Jin Y et al (2012) Dwarf and low-tillering acts as a direct downstream target of a GSK3/shaggy-like kinase to mediate brassinosteroid responses in rice. Plant Cell 24:2562–2577
Sui P, Jin J, Ye S et al (2012) H3k36 methylation is critical for brassinosteroid regulated plant growth and development in rice. Plant J 70:340–347
Toki S, Hara N, Ono K et al (2006) Early infection of scutellum tissue with agrobacterium allows high-speed transformation of rice. Plant J 47:969–976
Duan K, Li L, Hu P et al (2006) A brassinolide-suppressed rice MADS-box transcription factor, OsMDP1, has a negative regulatory role in BR signaling. Plant J 47:519–531
Chory J, Nagpal P, Peto CA (1991) Phenotypic and genetic analysis of det2, a new mutant that affects light-regulated seedling development in Arabidopsis. Plant Cell 3:445–459
Fujioka S, Noguchi T, Takatsuto S et al (1998) Activity of brassinosteroids in the dwarf rice lamina inclination bioassay. Phytochemistry 49:1841–1848
Jiang Y, Bao L, Jeong SY et al (2012) XIAO is involved in the control of organ size by contributing to the regulation of signaling and homeostasis of brassinosteroids and cell cycling in rice. Plant J 70:398–408
Je BI, Piao HL, Park SJ et al (2010) Rav-like1 maintains brassinosteroid homeostasis via the coordinated activation of BRI1 and biosynthetic genes in rice. Plant Cell 22:1777–1791
He J-X, Gendron JM, Yang Y et al (2002) The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proc Natl Acad Sci USA 99:10185–10190
Wang L, Xu Y, Zhang C et al (2008) OsLIC, a novel CCCH-type zinc finger protein with transcription activation, mediates rice architecture via brassinosteroids signaling. PLoS ONE 3:e3521
Zhang L-Y, Bai M-Y, Wu J et al (2009) Antagonistic HLH/bHLH transcription factors mediate brassinosteroid regulation of cell elongation and plant development in rice and Arabidopsis. Plant Cell 21:3767–3780
Symons GM, Ross JJ, Jager CE et al (2008) Brassinosteroid transport. J Exp Bot 59:17–24
Wang Z-Y, Nakano T, Gendron J et al (2002) Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev Cell 2:505–513
Iwata N, Saton H, Omura T (1984) The relationships between chromosomes identified cytologically and linkage groups. Rice Genet Newsl 1:128–132
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31201194, 31171532, 91335105 and 31201183).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Zhenyuan Shi, Yuchun Rao and Jie Xu contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Shi, Z., Rao, Y., Xu, J. et al. Characterization and cloning of SMALL GRAIN 4, a novel DWARF11 allele that affects brassinosteroid biosynthesis in rice. Sci. Bull. 60, 905–915 (2015). https://doi.org/10.1007/s11434-015-0798-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-015-0798-8