Abstract
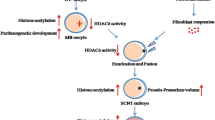
Although the somatic cell nuclear transfer (SCNT) technique has been used extensively for cloning and generating transgenic pigs, the cloning efficiency is still very low. It has been proposed that the low efficiency of this technique is the result of incomplete epigenetic reprogramming and abnormal gene expression during early embryonic development. In this study, we investigate the effect of Scriptaid, a low-toxicity histone deacetylase inhibitor, on the developmental competence of porcine SCNT embryos. We found that treating SCNT embryos with 500 nmol/L Scriptaid for 15 h after activation significantly enhanced the blastocyst formation rate (27.7%) compared with the untreated group (control) (12.2%, P<0.05). Using an immunofluorescence technique to measure the average fluorescence intensity, we also found that treating SCNT embryos with Scriptaid increased the level of histone acetylation on histone H3 at lysine 14 (acH3K14). Furthermore, treating embryos with Scriptaid increased the expression level of three genes that play important roles during embryonic development (Oct4, Klf4 at the blastocyst stage and Nanog at the 4-cell stage). Moreover, the expression level of the apoptosis-related gene Caspase-3 was significantly lower in the Scriptaid-treated SCNT embryos compared with the control SCNT embryos at the 4-cell and blastocyst stages. In conclusion, these results indicate that Scriptaid treatment improves the development and nuclear reprogramming of porcine SCNT embryos.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Polejaeva I A, Chen S H, Vaught T D, et al. Cloned pigs produced by nuclear transfer from adult somatic cells. Nature, 2000, 407: 86–90
Onishi A, Iwamoto M, Akita T, et al. Pig cloning by microinjection of fetal fibroblast nuclei. Science, 2000, 289: 1188–1190
Lai L, Kolber-Simonds D, Park K W, et al. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science, 2002, 295: 1089–1092
Whitworth K M, Prather R S. Somatic cell nuclear transfer efficiency: How can it be improved through nuclear remodeling and reprogramming? Mol Reprod Dev, 2010, 77: 1001–1015
Cezar G G. Epigenetic reprogramming of cloned animals. Cloning Stem Cells, 2003, 5: 165–180
Jeanisch R, Eggan K, Humpherys D, et al. Nuclear cloning, stem cells, and genomic reprogramming. Cloning Stem Cells, 2002, 4: 389–396
Bird A. DNA methylation patterns and epigenetic memory. Genes Dev, 2002, 16: 6–21
Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet, 2002, 3: 662–673
Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev, 1998, 12: 599–606
Oliveira C S, Saraiva N Z, de Souza M M, et al. Effects of histone hyperacetylation on the preimplantation development of male and female bovine embryos. Reprod Fertil Dev, 2010, 22: 1041–1048
Miyoshi K, Mori H, Mizobe Y, et al. Valproic acid enhances in vitro development and Oct-3/4 expression of miniature pig somatic cell nuclear transfer embryos. Cell Reprogram, 2010, 12: 67–74
Huang Y, Tang X, Xie W, et al. Histone deacetylase inhibitor significantly improved the cloning efficiency of porcine somatic cell nuclear transfer embryos. Cell Reprogram, 2011, 13: 513–520
Kishigami S, Mizutani E, Ohta H, et al. Significant improvement of mouse cloning technique by treatment with trichostatin A after somatic nuclear transfer. Biochem Biophys Res Commun, 2006, 340: 183–189
Akagi S, Matsukawa K, Mizutani E, et al. Treatment with a histone deacetylase inhibitor after nuclear transfer improves the preimplantation development of cloned bovine embryos. J Reprod Dev, 2011, 57: 120–126
Yamanaka K, Sugimura S, Wakai T, et al. Acetylation level of histone H3 in early embryonic stages affects subsequent development of miniature pig somatic cell nuclear transfer embryos. J Reprod Dev, 2009, 55: 638–644
Zhao J, Ross J W, Hao Y, et al. Significant improvement in cloning efficiency of an inbred miniature pig by histone deacetylase inhibitor treatment after somatic cell nuclear transfer. Biol Reprod, 2009, 81: 525–530
Wu X, Li Y, Li G P, et al. Trichostatin A improved epigenetic modifications of transfected cells but did not improve subsequent cloned embryo development. Anim Biotechnol, 2008, 19: 211–224
Whitworth K M, Zhao J, Spate L D, et al. Scriptaid corrects gene expression of a few aberrantly reprogrammed transcripts in nuclear transfer pig blastocyst stage embryos. Cell Reprogram, 2011, 13: 191–204
Van Thuan N, Bui H T, Kim J H, et al. The histone deacetylase inhibitor scriptaid enhances nascent mRNA production and rescues full-term development in cloned inbred mice. Reproduction, 2009, 138: 309–317
Zhao J, Hao Y, Ross J W, et al. Histone deacetylase inhibitors improve in vitro and in vivo developmental competence of somatic cell nuclear transfer porcine embryos. Cell Reprogram, 2010, 12: 75–83
Wang L J, Zhang H, Wang Y S, et al. Scriptaid improves in vitro development and nuclear reprogramming of somatic cell nuclear transfer bovine embryos. Cell Reprogram, 2011, 13: 431–439
Lai L, Prather R S. Production of cloned pigs by using somatic cells as donors. Cloning Stem Cells, 2003, 5: 233–241
Fan H Y, Sun Q Y. In vitro maturation and fertilization of pig oocytes. Methods Mol Biol, 2004, 253: 227–234
Tang F, Barbacioru C, Nordman E, et al. RNA-Seq analysis to capture the transcriptome landscape of a single cell. Nat Protoc, 2010, 5: 516–535
Santos F, Zakhartchenko V, Stojkovic M, et al. Epigenetic marking correlates with developmental potential in cloned bovine preimplantation embryos. Curr Biol, 2003, 13: 1116–1121
Zlatanova J, Caiafa P, Van Holde K. Linker histone binding and displacement: Versatile mechanism for transcriptional regulation. FASEB J, 2000, 14: 1697–1704
Hong L, Schroth G P, Matthews H R, et al. Studies of the DNA binding properties of histone H4 amino terminus. Thermal denaturation studies reveal that acetylation markedly reduces the binding constant of the H4 “tail” to DNA. J Biol Chem, 1993, 268: 305–314
Armstrong L, Lako M, Dean W, et al. Epigenetic modification is central to genome reprogramming in somatic cell nuclear transfer. Stem Cells, 2006, 24: 805–814
Cervoni N, Szyf M. Demethylase activity is directed by histone acetylation. J Biol Chem, 2001, 276: 40778–40787
Bui H T, Seo H J, Park M R, et al. Histone deacetylase inhibition improves activation of ribosomal RNA genes and embryonic nucleolar reprogramming in cloned mouse embryos. Biol Reprod, 2011, 85: 1048–1056
Costa-Borges N, Santalo J, Ibanez E. Comparison between the effects of Valproic Acid and Trichostatin A on the in vitro development, blastocyst quality, and full-term development of mouse somatic cell nuclear transfer embryos. Cell Reprogram, 2010, 12: 437–446
Kurosaka S, Eckardt S, McLaughlin K J. Pluripotent lineage definition in bovine embryos by Oct4 transcript localization. Biol Reprod, 2004, 71: 1578–1582
Niwa H, Miyazaki J, Smith A G. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet, 2000, 24: 372–376
Rodda D J, Chew J L, Lim L H, et al. Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem, 2005, 280: 24731–24737
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 2006, 126: 663–676
du Puy L, Lopes S M, Haagsman H P, et al. Analysis of coexpression of OCT4, NANOG and SOX2 in pluripotent cells of the porcine embryo, in vivo and in vitro. Theriogenology, 2011, 75: 513–526
Su J M, Wang Y S, Li Y Y, et al. Oxamflatin significantly improves nuclear reprogramming, blastocyst quality, and in vitro development of bovine SCNT embryos. PLoS One, 2011, 6: e23805
Cervera R P, Marti-Gutierrez N, Escorihuela E, et al. Trichostatin A affects histone acetylation and gene expression in porcine somatic cell nucleus transfer embryos. Theriogenology, 2009, 72: 1097–1110
Haouzi D, Hamamah S. Pertinence of apoptosis markers for the improvement of in vitro fertilization (IVF). Curr Med Chem, 2009, 16: 1905–1916
Meng Q, Polgar Z, Liu J, et al. Live birth of somatic cell-cloned rabbits following trichostatin A treatment and cotransfer of parthenogenetic embryos. Cloning Stem Cells, 2009, 11: 203–208
Martinez-Diaz M A, Che L, Albornoz M, et al. Pre- and postimplantation development of swine-cloned embryos derived from fibroblasts and bone marrow cells after inhibition of histone deacetylases. Cell Reprogram, 2010, 12: 85–94
Sangalli J R, De Bem T H, Perecin F, et al. Treatment of nucleardonor cells or cloned zygotes with chromatin-modifying agents increases histone acetylation but does not improve full-term development of cloned cattle. Cell Reprogram, 2012, 14: 235–247
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
This article is published with open access at Springerlink.com
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Zhou, Y., Huang, Y., Xie, W. et al. Scriptaid affects histone acetylation and the expression of development-related genes at different stages of porcine somatic cell nuclear transfer embryo during early development. Chin. Sci. Bull. 58, 2044–2052 (2013). https://doi.org/10.1007/s11434-013-5827-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-013-5827-x