Abstract
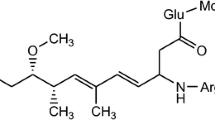
In order to investigate the catalytic performance of anodic TiO2 nanotubes and their practical application in the treatment of refractory microcystins (MCs) in natural-water samples, TiO2 nanotubes of diameter of 50–80 nm were fabricated by anodization in C2H2O4·2H2O containing NH4F. Under irradiation with natural sunlight, MC-LR was totally degraded after 1 d using the anodic TiO2 nanotubes. In contrast, the removal efficiency without TiO2 nanotubes was as low as 47.7% within 20 d. In addition, a mixture of anatase and rutile TiO2 gave higher photocatalytic activity than the single phase did. The pH also influenced the adsorption capacity of the TiO2 nanotubes. The order of MC-LR degradation efficiencies at different pH values was 3.5 > 8.0 > 10.0. After five repeated experiments on the degradation of MC-LR for 7 h, the degradation efficiency was still stable.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Linsebigler A L, Lu G Q, Yates J T Jr. Photocatalysis on TiO2 surfaces: Principles mechanisms and selected results. Chem Rev, 1995, 95: 735–758
Robertson P K J. Semiconductor photocatalysis: An environmentally acceptable alternative production technique and effluent treatment process. J Clean Prod, 1996, 3–4: 203–212
Liu I, Lawton L A, Cornish B, et al. Mechanistic and toxicity studies of the photocatalytic oxidation of microcystin-LR. J Photochem Photobiol A-Chem, 2002, 148: 349–354
Liu I, Lawton L A, Robertson P K J. Mechanistic studies of the photocatalytic oxidation of microcystin-LR: An investigation of byproducts of the decomposition process. Environ Sci Technol, 2003, 37: 3214–3219
Antoniou M G, Shoemaker J A, De la Cruz A A, et al. LC/MS/MS structure elucidation of reaction intermediates formed during the TiO2 photocatalysis of microcystin-LR. Toxicon, 2008, 51: 1103–1118
Sopyan I, Watanabe M, Murasawa S, et al. An efficient TiO2 thin-film photocatalyst: Photocatalytic properties in gas-phase acetaldehyde degradation. J Photochem Photobiol A-Chem, 1996, 98: 79–86
Quan X, Yang S G, Ruan X L, et al. Preparation of titania nanotubes and their environmental application as electrode. Environ Sci Technol, 2005, 39: 3770–3775
Macak J M, Tsuchiya H, Schmuki P. High-aspect-ratio TiO2 nanotubes by anodization of titanium. Angew Chem Int Ed, 2005, 44: 2100–2102
Liu N, Lee K Y, Schmuki P. Small diameter TiO2 nanotubes vs. nanopores in dye sensitized solar cells. Electrochem Commun, 2012, 15: 1–4
Hahn R, Macak J M, Schmuki P. Rapid anodic growth of TiO2 and WO3 nanotubes in fluoride free electrolytes. Electrochem Commun, 2007, 9: 947–952
Su Y L, Zhang X W, Han S, et al. F-B-codoping of anodized TiO2 nanotubes using chemical vapor deposition. Electrochem Commun, 2007, 9: 2291–2298
Falconer I R, Burch M D, Steffensen D A, et al. Toxicity of the blue-alga (cyannobacteriun) Micocystis aeruginosa in drinking water to growing pigs, as an animal model for human injury and risk assessment. Environ Toxicol Water Qual, 1994, 9: 131–139
Keijola A M, Himberg K, Esala A L, et al. Removal of cyanobacterial toxins in water treatment processes: Laboratory and pilot-scale experiments. Toxic Assess, 1988, 3: 643–656
Pérez S, Aga D S. Liquid chromatography tandem mass spectrometric analysis and environmental fate of microcystins in water. Trac-Trends Anal Chem, 2005, 24: 658–670
Edwards C, Lawton L A, Coyle S M, et al. Laboratory-scale purification of microcystins using flash chromatography and reversed-phase high-performance liquid chromatography. J Chromatogr A, 1996, 734: 163–173
Su Y L, Zhang X W, Zhou M H, et al. Preparation of high efficient photoelectrode of N-F-codoped TiO2 nanotubes. J Photochem Photobiol A-Chem, 2008, 194: 152–160
Watanabe T A, Nakajima R, Wang R, et al. Photocatalytic activity and photoinduced hydrophilicity of titanium dioxide coated glass. Thin Solid Films, 1999, 351: 260–263
Vittadni A, Selloni A, Rotzinger F P, et al. Structure and energetics of water adsorbed at TiO2 anatase (101) and (001) surfaces. Phys Rev Lett, 1998, 81: 2954–2957
Ruan C M, Paulose M, Varghese O K, et al. Fabrication of highly ordered TiO2 nanotube arrays using an organic electrolyte. J Phys Chem B, 2005, 109: 15754–15759
Zhuang H F, Lin C J, Lai Y K, et al. Some critical structure factors of titanium oxide nanotube array in its photocatalytic activity. Environ Sci Technol, 2007, 41: 4735–4740
Berger S, Tsuchiya H, Ghicov A, et al. High photocurrent conversion efficiency in self-organized porous WO3. Appl Phys Lett, 2006, 88: 203119–203121
Mor G K, Shankar K, Paulose M, et al. Use of highly-ordered TiO2 nanotube arrays in dye-sensitized solar cells. Nano Lett, 2006, 6: 215–218
Park J H, Kim S, Bard A J. Novel carbon-doped TiO2 nano-tube arrays with high aspect ratios for efficient solar water splitting. Nano Lett, 2006, 6: 24–28
Sclafani A, Palmisano L, Schiavello M. Difference of the preparation methods of TiO2 on the photocatalytic degradation of phenol in aqueous dispersion. J Phys Chem, 1990, 94: 829–832
Bendavid A, Martin P J, Jamting A, et al. Structural and optical properties of titanium oxide thin films deposited by filtered arc deposition. Thin Solid Films, 1999, 356: 6–11
Bacsa R R, Kiwi J. Effect of rutile phase on the photocatalytic properties of nanocrystalline titania during the degradation of p-coumaric acid. Appl Catal B-Environ, 1998, 16: 19–29
Van der Meulen T, Mattson A, Österlund L. A comparative study of the photocatalytic oxidation of propane on anatase, rutile, and mixed-phase anatase-rutile TiO2 nanoparticles: Role of surface intermediates. J Catal, 2007, 251: 131–144
Antoniou M G. Mechanistic studies on the degradation of cyanobacterial toxins and other nitrogen containing compounds with hydroxyl and sulfate radical based advanced oxidation technologies. Doctor Dissertation. Cincinnati: University of Cincinati, 2010
Lawton L A, Robertson P K J, Cornish B J P A, et al. Processes influencing surface interaction and photocatalytic destruction of microcystins on titanium dioxide photocatalysts. J Catal, 2003, 213: 109–113
Ward M D, White J R, Bard A J. Electrochemical investigation of the energetics of particulate titanium dioxide photocatalysts. The methyl viologen-acetate system. J Am Chem Soc, 1983, 105: 27–31
Schwarz J A. Methods for preparation of catalytic materials. Chem Rev, 1995, 95: 477–510
Harada K, Tsuji K, Watanabe M F, et al. Stability of microcystins from cyanobacteria-III. Effect of pH and temperature. Phycologia, 1996, 35: 83–88
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published with open access at Springerlink.com
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Su, Y., Deng, Y., Zhao, L. et al. Photocatalytic degradation of microcystin-LR using TiO2 nanotubes under irradiation with UV and natural sunlight. Chin. Sci. Bull. 58, 1156–1161 (2013). https://doi.org/10.1007/s11434-012-5637-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-012-5637-6