Abstract
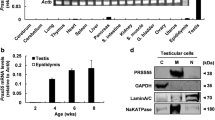
Proteases in male reproductive tract are considered to play key roles in fertilization processes. In contrast to mammals, there are limited data concerning crustacean sperm proteases. We previously identified a novel sperm gelatinase (MSG) from Macrobrachium rosenbergii that was inhibited by a male reproduction-related Kazal-type protease inhibitor (MRPINK) specifically. In the present study, MSG was found to be distributed on the vas deferens and terminal ampullae by Western blot. Immunohistochemical analyses revealed that MSG was expressed in secretory epithelial cells and sperm distributed mainly at the base zone. RNA interference (RNAi) mediated knock-down of MSG resulted in a marked loss of sperm gelatinolytic activity. Taken together, the results show that MSG is closely linked to and probably involved in the fertilization process.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Deppe M, Morales P, Sanchez R. Effect of protease inhibitors on the acrosome reaction and sperm-zona pellucida binding in bovine sperm. Reprod Domest Anim, 2008, 43: 713–719
Baba T, Azuma S, Kashiwabara S, et al. Sperm from mice carrying a targeted mutation of the acrosin gene can penetrate the oocyte zona pellucida and effect fertilization. J Biol Chem, 1994, 269: 31845–31849
Honda A, Yamagata K, Sugiura S, et al. A mouse serine protease TESP5 is selectively included into lipid rafts of sperm membrane presumably as a glycosylphosphatidylinositol-anchored protein. J Biol Chem, 2002, 277: 16976–16984
Uhrin P, Schofer C, Zaujec J, et al. Male fertility and protein C inhibitor/plasminogen activator inhibitor-3 (PCI): Localization of PCI in mouse testis and failure of single plasminogen activator knockout to restore spermatogenesis in PCI-deficient mice. Fertil Steril, 2007, 88: 1049–1057
Yamagata K, Murayama K, Okabe M, et al. Acrosin accelerates the dispersal of sperm acrosomal proteins during acrosome reaction. J Biol Chem, 1998, 273: 10470–10474
Yamashita M, Honda A, Ogura A, et al. Reduced fertility of mouse epididymal sperm lacking Prss21/Tesp5 is rescued by sperm exposure to uterine microenvironment. Genes Cells, 2008, 13: 1001–1013
Slowinska M, Olczak M, Liszewska E, et al. Isolation, characterization and cDNA sequencing of acrosin from turkey spermatozoa. Comp Biochem Physiol B, 2010, 157: 127–136
Ciereszko A, Dabrowski K, Mims S D, et al. Characteristics of sperm acrosin-like activity of paddlefish (Polyodon spathula Walbaum). Comp Biochem Physiol B, 2000, 125: 197–203
Sawada H. Ascidian sperm lysin system. Zoolog Sci, 2002, 19: 139–151
Rios M, Barros C. Trypsin-like enzymes during fertilization in the shrimp Rhynchocinetes typus. Mol Reprod Dev, 1997, 46: 581–586
Li Y, Ma W M, Dai J Q, et al. Inhibition of a novel sperm gelatinase in prawn sperm by the male reproduction-related Kazal-type peptidase inhibitor. Mol Reprod Dev, 2008, 75: 1327–1337
Kodama E, Baba T, Kohno N, et al. Spermosin, a trypsin-like protease from ascidian sperm: cDNA cloning, protein structures and functional analysis. Eur J Biochem, 2002, 269: 657–663
Yanagimachi R. Mammalian fertilization. In: Knobil E, Neill J D, eds. Physiology of Reproduction, 2nd edn. New York: Raven Press, 1994. A189–A317
Stein K K, Go J C, Lane W S, et al. Proteomic analysis of sperm regions that mediate sperm-egg interactions. Proteomics, 2006, 6: 3533–3543
Lynn J W, Clark W H Jr. A morphological examination of sperm-egg interaction in the freshwater prawn, Macrobrachium rosenbergii. Biol Bull, 1983, 164: 446–458
Cao J X, Dai J Q, Dai Z M, et al. A male reproduction-related Kazal-type peptidase inhibitor gene in the prawn, Macrobrachium rosenbergii: Molecular characterization and expression patterns. Mar Biotechnol, 2007, 9: 45–55
Yodmuang S, Tirasophon W, Roshorm Y, et al. YHV-protease dsRNA inhibits YHV replication in Penaeus monodon and prevents mortality. Biochem Biophys Res Commun, 2006, 341: 351–356
Liu D Y, Baker H W. Inhibition of acrosin activity with a trypsin inhibitor blocks human sperm penetration of the zona pellucida. Biol Reprod, 1993, 48: 340–348
Lynn J W, Clark W H Jr. The fine structure of the mature sperm of the freshwater prawn, Macrobrachium rosenbergii. Biol Bull, 1983, 164: 459–470
Dacheux J L, Castella S, Gatti J L, et al. Epididymal cell secretory acitivities and the role of proteins in boar sperm maturation. Theriogenology, 2005, 63: 319–341
Cesari A, Monclus Mde L, Tejon G P, et al. Regulated serine proteinase lytic system on mammalian sperm surface: There must be a role. Theriogenology, 2010, 74: 699–711
Kawano N, Kang W, Yamashita M, et al. Mice lacking two sperm serine proteases, ACR and PRSS21, are subfertile, but the mutant sperm are infertile in vitro. Biol Reprod, 2010, 83: 359–369
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
This article is published with open access at Springerlink.com
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Yang, F., Qian, Y., Ma, W. et al. MSG is involved in sperm gelatinolytic activity in the prawn, Macrobrachium rosenbergii . Chin. Sci. Bull. 58, 2113–2118 (2013). https://doi.org/10.1007/s11434-012-5597-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-012-5597-x