Abstract
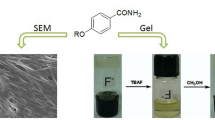
Two poly(benzyl ether) dendrons, decorated in their periphery with nitrile groups, were divergently synthesized and fully characterized. Their gelation properties were studied by using scanning electron microscopy (SEM), X-ray crystal structure analysis and concentration- and temperature-dependent 1H NMR spectroscopy. It was found that the gelation capability of these dendrons was highly dependent on dendron generation, and the second-generation dendron G2-CN proved to be highly efficient organogelator despite the lacking of any conventional gelation motifs, such as amides, long alkyl side chains and steroidal groups. The multiple strong π-π stacking interactions and hydrogen bonding interactions due to the peripheral isophthalonitrile motifs were proved to be the main driving forces to form the self-assembled gel.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
Hirst A R, Smith D K. Dendritic gelators. Top Curr Chem, 2005, 256: 237–373
Smith D K. Dendritic gels: Many arms make light work. Adv Mater, 2006, 18: 2773–2778
Grinstaff M W. Dendritic macromers for hydrogel formation: Tailored materials for ophthalmic, orthopedic, and biotech applications. J Poly Sci A-Poly Chem, 2008, 46: 383–400
Newkome G R, Baker G R, Saunders M J, et al. Two-directional cascade molecules: Synthesis and characterization of [9]-n-[9] a rborols. Chem Soc Chem Commun, 1986: 752–753
Newkome G R, Baker G R, Arai S, et al. Synthesis and characterization of two-directional cascade molecules and formation of aqueous gels. J Am Chem Soc, 1990, 112: 8458–8465
Marmillon C, Gauffre F, Majoral J P, et al. Organophosphorus dendrimers as new gelators for hydrogels. Angew Chem Int Ed, 2001, 40: 2626–2629
Jang W D, Jiang D L, Aida T. Dendritic physical gel: Hierarchical self-organization of a peptide-core dendrimer to form a micrometer-scale fibrous assembly. J Am Chem Soc, 2000, 122: 3232–3233
Zubarev E R, Pralle M U, Stupp S I, et al. Self-assembly of dendron rod-coil molecules into nanoribbons. J Am Chem Soc, 2001, 123: 4105–4106
Kim C, Kim K T, Chang Y, et al. Supramolecular assembly of amide dendrons. J Am Chem Soc, 2001, 123: 5586–5587
Partridge K S, Smith D K, Dykes G M, et al. Supramolecular dendritic two-component gel. Chem Commun, 2001, 319-320
Huang B Q, Hirst A R, Smith D K, et al. A direct comparison of one- and two-component dendritic self-assembled materials: Elucidating molecular recognition pathways. J Am Chem Soc, 2005, 127: 7130–7139
Ji Y, Luo Y F, Jia X R, et al. A dendron based on natural amino acids: Synthesis and behavior as an organogelator and lyotropic liquid crystal. Angew Chem Int Ed, 2005, 44: 6025–6029
Kuang G C, Ji Y, Jia X R, et al. Self-assembly of amino-acid-based dendrons: Organogels and lyotropic and thermotropic liquid crystals. Chem Mater, 2008, 20: 4173–4175
Kuang G C, Jia X R, Chen E Q, et al. Organogels and liquid crystalline properties of amino acid-based dendrons: A systematic study on structure-property relationship. Chem Mater, 2012, 24: 71–80
Chow H F, Zhang J. Structural diversity of a-amino acid based layer-block dendrons and their layer-block sequence-dependent gelation properties. Chem Eur J, 2005, 11: 5817–5831
Lau K N, Chow H F, Chan M C, et al. Dendronized polymer organo gels from click chemistry: A remarkable gelation property owing to synergistic functional-group binding and dendritic size effects. Angew Chem Int Ed, 2008, 47: 6912–6916
Percec V, Peterca M, Yurchenko M E, et al. Thixotropic twin-dendritic organogelators. Chem Eur J, 2008, 14: 909–918
Yang M, Wang W, Wegner G, et al. Self-assembled structures in organogels of amphiphilic diblock codendrimers. Chem Eur J, 2008, 14: 3330–3337
Duan P F, Liu M H. Design and self-assembly of L-glutamate-based aromatic dendrons as ambidextrous gelators of water and organic solvents. Langmuir 2009, 25: 8706–8713
Chen Y L, Bo Z S, Liu C Y, et al. Dendritic effect on supramolecular self-assembly: Organogels with strong fluorescence emission induced by aggregation. Langmuir, 2009, 25: 8548–8555
Seo M, Kim J H, Kim S Y, et al. Self-association of bis-dendritic organogelators: The effect of dendritic architecture on multivalent cooperative interactions. Chem Eur J, 2010, 16: 2427–2441
Yang X C, Lu R, Gai F Y. Rigid dendritic gelators based on oligocarbazoles. Chem Commun, 2010, 46: 1088–1090
Rajamalli P, Prasad E. Luminescent micro and nanogel formation from AB3 type poly(aryl ether) dendron derivatives without conventional multi-interactive gelation motifs. New J Chem, 2011, 35: 1541–1548
Feng Y, He Y M, Fan Q H, et al. Peripherally dimethyl isophthalate-functionalized poly(benzyl ether) dendrons: A new kind of unprecedented highly efficient organogelators. J Am Chem Soc, 2009, 131: 7950–7951
Chen Q, Zhang D Q, Fan Q H, et al. Light-triggered self-assembly of a spiropyran-functionalized dendron into nano-/micrometer-sized particles and photoresponsive organogel with switchable fluorescence. Adv Funct Mater, 2010, 20: 36–42
Barclay T M, Cordes A W, Oakley R T, et al. Oligothiophenes end-capped by nitriles. Preparation and crystal structures of π,π-dicyanooligothiophenes NC(C4H2S)nCN (n=3–6). Chem Mater, 1997, 9: 981–990
Nishida J, Shio Murai N, Yamashita Y, et al. Preparation, characterization, and FET properties of novel dicyanopyrazinoquinoxaline derivatives. Org Lett, 2004, 6: 2007–2010
Jang K, Kinyanjui J M, Lee D C, et al. Morphological control of one-dimensional nanostructures of t-shaped asymmetric bisphenazine. Chem Mater, 2009, 21: 2070–2076
Eldridge J E, Ferry J D. Studies of the cross-linking process in gelatin gels. III. Dependence of melting point on concentration and molecular weight. J Phys Chem, 1954, 58: 992–995
Feng Y, He Y M, Fan Q H, et al. A liquid-phase approach to functionalized janus dendrimers: Novel soluble supports for organic synthesis. Org Lett, 2007, 9: 2261–2264
Fernández G, Sánchez L, Martín N, et al. Large exTTF-based dendrimers. Self-assembly and peripheral cooperative multiencapsulation of C60. J Am Chem Soc, 2008, 130: 10674–10683
Yang H, Yi T, Li F Y, et al. Switchable fluorescent organogels and mesomorphic superstructure based on naphthalene derivatives. Langmuir, 2007, 23: 8224–8230
Author information
Authors and Affiliations
Corresponding authors
Additional information
This article is published with open access at Springerlink.com
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Feng, Y., Liu, Z., Wang, L. et al. Poly(benzyl ether) dendrons without conventional gelation motifs as a new kind of effective organogelators. Chin. Sci. Bull. 57, 4289–4295 (2012). https://doi.org/10.1007/s11434-012-5479-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-012-5479-2