Abstract
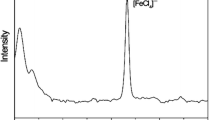
1,3-Bis(2,6-diisopropylphenyl)imidazolium chloride, [DIPrim]Cl, was used to produce a novel iron(III)-containing imidazolium salt [DIPrim][FeCl4], which included a N,N-diarylimidazolium cation (R = 2,6-diisopropylphenyl), [DIPrim]+, and tetrachloroferrate(III) anion, [FeCl4]−. This compound was an effective and easy-to-use catalyst for the cross-coupling of aryl Grignard reagents with primary and secondary alkyl halides bearing β-hydrogens. After simply decanting the cross-coupling product in the ether layer, [DIPrim][FeCl4] could be reused in at least four successive runs without significant loss of catalytic activity.
Article PDF
Similar content being viewed by others
Avoid common mistakes on your manuscript.
References
de Meijere A, Diederich F. Metal-catalyzed Cross-coupling Reactions. 2nd ed. Wiley-VCH: Weinheim, 2004
Sherry B D, Fürstner A. The promise and challenge of iron-catalyzed cross coupling. Acc Chem Res, 2008, 41: 1500–1511
Czaplik W M, Mayer M, Cvengroš J, et al. Coming of age: Sustainable iron-catalyzed cross-coupling reactions. ChemSusChem, 2009, 2: 396–417
Martin R, Fürstner A. Cross-coupling of alkyl halides with aryl Grignard reagents catalyzed by a low-valent iron complex. Angew Chem Int Ed, 2004, 43: 3955–3957
Bedford R B, Bruce D W, Frost R M, et al. Iron(III) salen-type catalysts for the cross-coupling of aryl Grignards with alkyl halides bearing β-hydrogens. Chem Commum, 2004, 2822–2823
Martin R B, Betham M, Bruce D W, et al. Iron nanoparticles in the coupling of alkyl halides with aryl Grignard reagents. Chem Commun, 2006, 1398–1400
Qian X, Dawe L N, Kozak C M. Catalytic alkylation of aryl Grignard reagents by iron(III) amine-bis(phenolated) complexes. Dalton Trans, 2011, 40: 933–943
Nagano T, Hayashi T. Iron-catalyzed Grignard cross-coupling with alkyl halides possessing β-hydrogens. Org Lett, 2004, 6: 1297–1299
Cahiez G, Habiak V, Duplais C, et al. Iron-catalyzed alkylations of aromatic Grignard reagents. Angew Chem Int Ed, 2007, 46: 4364–4366
Nakamura M, Matsuo K, Ito S, et al. Iron-catalyzed cross-coupling of primary and secondary alkyl halides with aryl Grignard reagents. J Am Chem Soc, 2004, 126: 3686–3687
Bedford R B, Bruce D W, Frost R M, et al. Simple iron-amine catalysts for the cross-coupling of aryl Grignards with alkyl halides bearing β-hydrogens. Chem Commun, 2005, 4161–4163
Bedford R B, Betham M, Bruce D W, et al. Iron-phosphine,-phosphite, -arsine, and -carbene catalysts for the coupling of primary and secondary alkyl halides with aryl Grignard reagents. J Org Chem, 2006, 71: 1104–1110
Buchwald S L, Bolm C. On the role of metal contaminants in cataly ses with FeCl3. Angew Chem Int Ed, 2009, 48: 5586–5587
Bica K, Gaertner P. An iron-containing ionic liquid as recyclable catalyst for aryl Grignard cross-coupling of alkyl halides. Org Lett, 2006, 8: 733–735
Alexznder M V, Khandekar A C, Samant S D. Sulfonylation reactions of aromatics using FeCl3-based ionic liquids. J Mol Catal A, 2004, 223: 75–83
Nguyen M D, Nguyen L V, Jeon E H, et al. Fe-containing ionic liquids as catalysts for the dimerization of bicycle[2.2.1]hepta-2,5-diene. J Catal, 2008, 258: 5–13
Wang H, Yan R Y, Li Z X, et al. Fe-containing magnetic ionic liquid as an effective catalyst for the glycolysis of poly(ethylene terephthalate). Catal Commun, 2010, 11: 763–767
Pârvulescu V I, Hardacre C. Catalysis in ionic liquids. Chem Rev, 2007, 107: 2615–2665
Šebesta R, Kmentová I, Toma Š. Catalysts with ionic tag and their use in ionic liquids. Green Chem, 2008, 10: 484–496
Gao H H, Yan C H, Tao X P, et al. Synthesis of anionic iron(II) complex bearing an N-heterocyclic carbene ligand and its catalysis for aryl Grignard cross-coupling of alkyl halides. Organometallics, 2010, 29: 4189–4192
Huang J K, Nolan S P. Efficient cross-coupling of aryl chlorides with aryl Grignard reagents (Kumada reaction) mediated by a palladium/imidazolium chloride system. J Am Chem Soc, 1999, 121: 9889–9890
Sitze M S, Schreiter E R, Patterson E V, et al. Ionic liquids based on FeCl3 and FeCl2. Raman scattering and ab initio calculation. Inorg Chem, 2001, 40: 2298–2304
Del Sesto R E, McCleskey T M, Burrel A K, et al. Structure and magnetic behavior of transition metal based ionic liquids. Chem Commun, 2008, 447–449
Author information
Authors and Affiliations
Corresponding authors
Additional information
This article is published with open access at Springerlink.com
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Yan, C., Wang, L., Gao, H. et al. An efficient and recyclable iron(III)-containing imidazolium salt catalyst for cross-coupling of aryl Grignard reagents with alkyl halides. Chin. Sci. Bull. 57, 1953–1958 (2012). https://doi.org/10.1007/s11434-011-4660-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-011-4660-3