Abstract
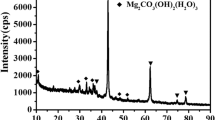
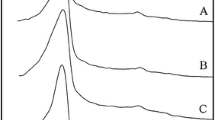
In this paper, a new delaminated mesoporous material was prepared by swelling the lamellar phyllosilicate Magadiite using cetyltrimethylammonium bromide and tetrapropylammonium hydroxide solution, followed by delamination under the cavitation of ultrasonic treatment. Various characterization tools including XRD, N2 adsorption, NMR, IR, SEM and TEM were employed to observe its structure and morphology. XRD results show that the delaminated material has no long-range crystalline order. Furthermore, not only high surface area is obtained, but almost half of it is external surface. The observation for the morphology further reveals that it is made up of the random stacking of single or few layers. All these characters show that this delaminated mesoporous material meets the requirements for application as support in the hydrotreatment of heavy molecules.
Similar content being viewed by others
References
Corma A. Inorganic solid acids and their use in acid-catalyzed hydrocarbon reactions. Chem Rev, 1995, 95: 559–614
Corma A. From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem Rev, 1997, 97: 2373–2419
Beck J S, Vartulli J C, Roth W J, et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J Am Chem Soc, 1992, 114: 10834–10843
Vartuli J C, Kresge C T, Leonowicz M E, et al. Synthesis of mesoporous materials: Liquid-crystal templating versus intercalation of layered silicates. Chem Mater, 1994, 6: 2070–2077
Beck J S, Vartulli J C, Kennedy G J, et al. Molecular or supramolecular templating: Defining the role of surfactant chemistry in the formation of microporous and mesoporous molecular sieves. Chem Mater,1994, 6: 1816–1821
Zhao D Y, Feng J L, Huo Q S. Triblock copolymer synthesis of mesoporous silica with periodic 50 to 300 angstrom pore. Science, 1998, 279: 448–552
Corma A, Fornes V, Pergher S B, et al. Delaminated zeolite precursors as selective acidic catalysts. Nature, 1998, 396: 353–356
Zubowa H L, Schneider M, Schreier E, et al. The influence of the expanding and exfoliating conditions on the structural transformation of the layered zeolite Nu-6(1). Microporous Mesoporous Mater, 2008, 109: 317–326
Maheshwari S, Jordan E, Kumar S, et al. Layer structure preservation during swelling, pillaring, and exfoliation of a zeolite precursor. J Am Chem Soc, 2008, 130: 1507–1516
Aguilara J, Pergherb S B C, Detonib C, et al. Alkylation of biphenyl with propylene using MCM-22 and ITQ-2 zeolites. Cata Today, 2008, 133-135: 667–672
Solsona B, Nieto J M L, Diaz U. Siliceous ITQ-6: A new support for vanadia in the oxidative dehydrogenation of propane. Microporous Mesoporous Mater, 2006, 94: 339–347
Corma A, Fornes V, Diaz U. ITQ-18 a new delaminated stable zeolite. Chem Commun, 2001, 24: 2642–2643
Zebib B, Lambert J F, Blanchard J, et al. LRS-1: A new delaminated phyllosilicate material with high acidity. Chem Mater, 2006, 18: 34–40
Shih S S, Vartuli J C. US Patent, 5 236 882, 1993-07-17
Kooli F, Li M H, Solhe F, et al. Characterization and thermal stability properties of intercalated Na-magadiite with cetyltrimethylammonium (C16TMA) surfactants. J Phys Chem Solids, 2006, 67:926–931
Wang Y R, Wang S F, Chang L C. hydrothermal synthesis of magadiite. Appl Clay Sci, 2006, 33: 73–77
Roth W J, Vartuli J C. Preparation of exfoliated zeolites from layered precursors:the role of pH and nature of intercalating media. Stud Surf Sci Catal, 2002, 141: 273–279
Graham G A, Robin K H, Kevin R F. A structural consideration of kanemite, octosilicate, magadiite and kenyaite. J Mater Chem, 1997, 7: 681–687
Gardiennet C, Tekely P. Structural and motional features of a layered sodium hydrous silicate as revealed by solid state NMR. J Phys Chem B, 2002, 106: 8928–8936
Sassi M, Miehé-Brendlé J, Patarin J, et al. Synthesis and characterization of montmorillonite-type phyllosilicates in a fluoride medium. Clay Miner, 2005, 40: 369–380
Rees L. Zeolites: Bond angles and chemical shifts. Nature, 1983, 303: 204–204
Engelhardt G, Luger S, Buhl J C, et al. 29Si MAS NMR of aluminosilicate sodalites: Correlations between chemical shifts and structure parameters. Zeolite, 1989, 9: 182–186
Huang Y, Jiang Z, Schwieger W. Vibrational spectroscopic studies of layered silicates. Chem Mater, 1999, 11: 1210–1217
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Bi, Y., Blanchard, J., Lambert, J.F. et al. Delamination of lamellar phyllosilicate Magadiite. Chin. Sci. Bull. 55, 2584–2588 (2010). https://doi.org/10.1007/s11434-010-3737-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-010-3737-8