Abstract
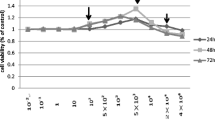
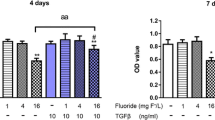
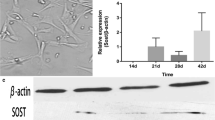
A series of experimental methods including 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H tetrazolium bromide (MTT) test, alkaline phosphatase (ALP) activity measurement, Oil Red O stain and measurement, mineralized function expression and quantitive real time RT-PCR (qRT-PCR) were employed to assess the effect of Nd3+ and Sm3+ on the proliferation, differentiation and mineralization function of primary osteoblasts (OBs) in vitro at cell and molecular levels. The experimental results suggest that concentration, culture time and ion species are pivotal factors for switching the biological effects of rare earth ions from toxicity to activity, from damage to protection, or from down-regulation to up-regulation.
Similar content being viewed by others
References
Bertini I, Lee Y M, Luchinat C, et al. Locating the metal ion in calcium-binding proteins by using cerium (III) as a probe. Chem Biochem, 2001, 2: 550–558
Chang J. Effects of lanthanum on the permeability of root plasmalemma and the absorption and accumulation of nutrients in rice and wheat. Plant Physiol Commun, 1991, 27: 17–21
Wang Z J, Liu D F, Lu P, et al. Ecological risk assessment, accumulation of rare earth elements in corn after agricultural application. J Environ Qual, 2001, 30: 37–45
Riggs B L. Pathogenesis of osteoporosis. AM J Obstet Gynecol, 1987, 156: 1342–1346
Suda T, Nakamura I, Jimi E, et al. Regulation of osteoclast function. J Bone Miner Res, 1997, 12: 869–879
Wattel A, Kamel S, Mentaverri R, et al. Potent inhibitory effect of naturally occurring flavonoids quercetin and kaemperol on in vitro osteoclastic bone resorption. Biochem Pharmacol, 2003, 65: 35–42
Jha A M, Singh A C. Clastogenicity of lanthanides-induction of micronuclei in root tips of vicia faba. Mutation Res, 1994, 322: 169–172
Quarles L D, Hartle J E, Middleton J P, et al. Aluminum-induced DNA synthesis in osteoblasts: mediation by a G-protein coupled cation sensing mechanism. J Cell Biochem, 1994, 56: 106–117
Hartle J E, Prpic V, Siddhanti S R, et al. Differential regulation of receptor-stimulated cyclic adenosine monophosphate production by polyvalent cations in MC3T3-E1 osteoblasts. J Bone Miner Res, 1996, 11: 789–799
Li R, Yang H, Wang K. La accumulation and microstructure change of leg bones of rats fed with La(NO3)3 in low dosage for long term. J Pek Univ (Health Sci), 2003, 35: 622–624
Zhang J C, Xu S J, Wang K, et al. Effects of the rare earth ions on bone resorbing function of rabbit mature osteoclasts in vitro. Chinese Sci Bull, 2003, 48: 2170–2175
Zhang J C, Li X X, Xu S J, et al. Effects of rare earth ions on proliferation, differentiation and function expression of cultured osteoblasts in vitro. Prog Nat Sci, 2004, 14: 404–409
Zhang D W, Zhang J C, Chen Y, et al. Effects of lanthanum and gadolinium on proliferation and differentiation of primary osteoblasts. Prog Nat Sci, 2007, 17: 618–623
Nishimori S, Tanaka Y, Chiba T, et al. Smad-mediated transcription is required for transforming growth factor-β1-induced p57Kip2 proteolysis in osteoblastic cells. J Biol Chem, 2001, 276: 10700–10705
Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods, 1983, 65: 55–63
Sekiya I, Larson B L, Smith J R, et al. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells, 2002, 20: 530–541
Gori F, Thomas T, Hicok K C, et al. Differentiation of human marrow stromal precursor cells: Bone morphogenetic protein-2 increases OSF2/CBFA1, enhances osteoblast commitment, and inhibits late adipocyte maturation. J Bone Miner Res, 1999, 14: 1522–1535
Winer J, Jung C K S, Shackel I, et al. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem, 1999, 270: 41–49
Wlodarski K H. Properties and origin of osteoblasts. Clin Orthoped Relat Res, 1990, 252: 276–293
Gimble J M. The function of adipocytes in the bone marrow stroma. New Biol, 1990, 2: 304–312
Nuttall M E, Gimble J M. Is there a therapeutic opportunity to either prevent or treat osteopenic disorders by inhibiting marrow adipogenesis? Bone, 2000, 27: 177–184
Ailhaud G, Grimaldi P, Negrel R. Cellular and molecular aspects of adi-pose tissue development. Annu Rev Nutr, 1992, 12: 207–233
Benayahu D, Zipori D, Wientroub S. Marrow adipocytes regulate growth and differentiation of osteoblasts. Biochem Biophys Res Comm, 1993, 197: 1245–1252
Sakaguchi K, Morita I, Murota S. Relationship between the ability to support differentiation of osteoclast-like cells and adipogenesis in murine stromal cells derived from bone marrow. Prostaglandins Leukotrienes and Essential Fatty Acids, 2000, 62: 319–327
Benayahu D, Peled A, Zipori D. Myeloblastic cell line expresses os-teoclastic properties following coculture with marrow stromal adi-pocytes. J Cell Biochem, 1994, 56: 374–384
Masaki N, Akira N, Kunikazu T, et al. Transcription factors and osteoblasts. Front Biosci, 1998, 3: 817–820
Rosen E D, Walkey C J, Puigserver P, et al. Transcriptional regulation of adipogenesis. Gene Dev, 2000, 14: 1293–1307
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Zhang, J., Shang, M., Zhang, D. et al. Effects of Nd3+ and Sm3+ on the proliferation, differentiation and mineralization function of primary osteoblasts in vitro . Chin. Sci. Bull. 55, 2505–2511 (2010). https://doi.org/10.1007/s11434-010-3153-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-010-3153-0