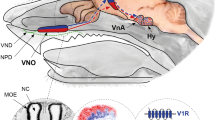
Abstract
The recognition and perception of chemical signals from environments are very important for the survival of organisms. In mammals, general chemical signals are mainly detected by the main olfactory system (MOS), while pheromones are primarily perceived by the vomeronasal system (VNS). Pheromones are chemicals released and recognized by individuals within the same species, which then induce physiological and behavioral changes in social and sexual activities. In this review, we focus on the recent advances on research in mammalian vomeronasal pheromone perception and those genetic components unique to vomeronasal signal transduction pathway, including vomeronasal receptor V1R and V2R gene families as well as transient receptor potential channel 2 gene (TRPC2), trying to shed light on further study of the molecular mechanisms of mammalian pheromone perception.
Similar content being viewed by others
References
Dulac C, Torello A T. Molecular detection of pheromone signals in mammals: From genes to behaviour. Nat Rev Neurosci, 2003, 4: 551–562
Karlson P, Luscher M. Pheromones: A new term for a class of biologically active substances. Nature, 1959, 183: 55–56
Keverne E B. The vomeronasal organ. Science, 1999, 286: 716–720
Schaal B, Coureaud G, Langlois D, et al. Chemical and behavioural characterization of the rabbit mammary pheromone. Nature, 2003, 424: 68–72
Liman E R, Corey D P, Dulac C. TRP2: A candidate transduction channel for mammalian pheromone sensory signaling. Proc Natl Acad Sci USA, 1999, 96: 5791–5796
Liberles S D, Horowitz L F, Kuang D, et al. Formyl peptide receptors are candidate chemosensory receptors in the vomeronasal organ. Proc Natl Acad Sci USA, 2009, 106: 9842–9847
Rivière S, Challet L, Fluegge D, et al. Formyl peptide receptor-like proteins are a novel family of vomeronasal chemosensors. Nature, 2009, 459: 574–577
Zufall F, Kelliher K R, Leinders-Zufall T. Pheromone detection by mammalian vomeronasal neurons. Microsc Res Tech, 2002, 58: 251–260
Loconto J, Papes F, Chang E, et al. Functional expression of murine V2R pheromone receptors involves selective association with the M10 and M1 families of MHC class Ib molecules. Cell, 2003, 112: 607–618
Dulac C, Axel R. A novel family of genes encoding putative pheromone receptors in mammals. Cell, 1995, 83: 195–206
Ryba N J, Tirindelli R. A new multigene family of putative pheromone receptors. Neuron, 1997, 19: 371–379
Herrada G, Dulac C. A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution. Cell, 1997, 90: 763–773
Matsunami H, Buck L B. A multigene family encoding a diverse array of putative pheromone receptors in mammals. Cell, 1997, 90: 775–784
Zhang J J, Webb D M. Evolutionary deterioration of the vomeronasal pheromone transduction pathway in catarrhine primates. Proc Natl Acad Sci USA, 2003, 100: 8337–8341
Liman E R, Innan H. Relaxed selective pressure on an essential component of pheromone transduction in primate evolution. Proc Natl Acad Sci USA, 2003, 100: 3328–3332
Young J M, Kambere M, Trask B J, et al. Divergent V1R repertoires in five species: Amplification in rodents, decimation in primates, and a surprisingly small repertoire in dogs. Genome Res, 2005, 15: 231–240
Grus W E, Shi P, Zhang J J. Largest vertebrate vomeronasal type 1 receptor gene repertoire in the semiaquatic platypus. Mol Biol Evol, 2007, 24: 2153–2157
Young J M, Trask B J. V2R gene families degenerated in primates, dog and cow, but expanded in opossum. Trends Genet, 2007, 23: 212–215
Jacobson L, Trotier D, Doving K B. Anatomical description of a new organ in the nose of domesticated animals by Ludvig Jacobson. Chem Senses, 1998, 23: 743–754
Vaccarezza O L, Sepich L N, Tramezzani J H. The vomeronasal organ of the rat. J Anat, 1981, 132: 167–185
Dulac C. Sensory coding of pheromone signals in mammals. Curr Opin Neurobiol, 2000, 10: 511–518
Bertmar G. Evolution of vomeronasal organs in vertebrates. Evolution, 1981, 35: 359–366
Eisthen H L. Evolution of vertebrate olfactory systems. Brain Behav Evol, 1997, 50: 222–233
Meisami E, Bhatnagar K P. Structure and diversity in mammalian accessory olfactory bulb. Microsc Res Tech, 1998, 43: 476–499
Vandenbergh J G, Whitsett J M, Lombardi J R. Partial isolation of a pheromone accelerating puberty in female mice. J Reprod Fertil, 1975, 43: 515–523
Singer A G, Macrides F, Clancy A N, et al. Purification and analysis of a proteinaceous aphrodisiac pheromone from hamster vaginal discharge. J Biol Chem, 1986, 261: 13323–13326
Cavaggioni A, Mucignat-Caretta C. Major urinary proteins, alpha (2U)-globulins and aphrodisin. Biochim Biophys Acta, 2000, 1482: 218–228
Bocskei Z, Groom C R, Flower D R, et al. Pheromone binding to two rodent urinary proteins revealed by X-ray crystallography. Nature, 1992, 360: 186–188
Bacchini A, Gaetani E, Cavaggioni A. Pheromone binding proteins of the mouse, Mus musculus. Experientia, 1992, 48: 419–421
Ma W, Miao Z, Novotny M V. Induction of estrus in grouped female mice (Mus domesticus) by synthetic analogues of preputial gland constituents. Chem Senses, 1999, 24: 289–293
Novotny M V, Ma W, Wiesler D, et al. Positive identification of the puberty-accelerating pheromone of the house mouse: The volatile ligands associating with the major urinary protein. Proc Biol Sci, 1999, 266: 2017–2022
Leinders-Zufall T, Lane A P, Puche A C, et al. Ultrasensitive pheromone detection by mammalian vomeronasal neurons. Nature, 2000, 405: 792–796
Chandrashekar J, Mueller K L, Hoon M A, et al. T2Rs function as bitter taste receptors. Cell, 2000, 100: 703–711
Del Punta K, Leinders-Zufall T, Rodriguez I, et al. Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature, 2002, 419: 70–74
Boschat C, Pelofi C, Randin O, et al. Pheromone detection mediated by a V1r vomeronasal receptor. Nat Neurosci, 2002, 5: 1261–1262
Pantages E, Dulac C. A novel family of candidate pheromone receptors in mammals. Neuron, 2000, 28: 835–845
Rodriguez I, Del Punta K, Rothman A, et al. Multiple new and isolated families within the mouse superfamily of V1r vomeronasal receptors. Nat Neurosci, 2002, 5: 134–140
Yang H, Shi P, Zhang Y P, et al. Composition and evolution of the V2r vomeronasal receptor gene repertoire in mice and rats. Genomics, 2005, 86: 306–315
Mombaerts P. Genes and ligands for odorant, vomeronasal and taste receptors. Nat Rev Neurosci, 2004, 5: 263–278
Clapham D E, Runnels L W, Strubing C. The TRP ion channel family. Nat Rev Neurosci, 2001, 2: 387–396
Minke B. The TRP channel and phospholipase C-mediated signaling. Cell Mol Neurobiol, 2001, 21: 629–643
Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev, 2002, 82: 429–472
Freichel M, Vennekens R, Olausson J, et al. Functional role of TRPC proteins in vivo: Lessons from TRPC-deficient mouse models. Biochem Biophys Res Commun, 2004, 322: 1352–1358
Vazquez G, Wedel B J, Aziz O, et al. The mammalian TRPC cation channels. Biochim Biophys Acta, 2004, 1742: 21–36
Wes P D, Chevesich J, Jeromin A, et al. TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci USA, 1995, 92: 9652–9656
Ranganathan R, Malicki D M, Zuker C S. Signal transduction in Drosophila photoreceptors. Annu Rev Neurosci, 1995, 18: 283–317
Niemeyer B A, Suzuki E, Scott K, et al. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell, 1996, 85: 651–659
Holy T E, Dulac C, Meister M. Responses of vomeronasal neurons to natural stimuli. Science, 2000, 289: 1569–1572
Harteneck C, Plant T D, Schultz G. From worm to man: Three subfamilies of TRP channels. Trends Neurosci, 2000, 23: 159–166
Spehr M, Hatt H, Wetzel C H. Arachidonic acid plays a role in rat vomeronasal signal transduction. J Neurosci, 2002, 22: 8429–8437
Grus W E, Zhang J J. Origin and evolution of the vertebrate Vomeronasal system viewed through system-specific genes. Bioessays, 2006, 28: 709–718
Grus W E, Zhang J J. Origin of the genetic components of the Vomeronasal system in the common ancestor of all extant vertebrates. Mol Biol Evol, 2009, 26: 407–419
Hoon M A, Adler E, Lindemeier J, et al. Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell, 1999, 96: 541–551
Keller A, Vosshall L B. Better smelling through genetics: Mammalian odor perception. Curr Opin Neurobiol, 2008, 18: 364–369
Grus WE, Shi P, Zhang Y P, et al. Dramatic variation of the vomeronasal pheromone receptor gene repertoire among five orders of placental and marsupial mammals. Proc Natl Acad Sci USA, 2005, 102: 5767–5772
Shi P, Zhang J J. Comparative genomic analysis identifies an evolutionary shift of Vomeronasal receptor gene repertoires in the vertebrate transition from water to land. Genome Res, 2007, 17: 166–174
Naito T, Saito Y, Yamamoto J, et al. Putative pheromone receptors related to the Ca2+-sensing receptor in Fugu. Proc Natl Acad Sci USA, 1998, 95: 5178–5181
Swaney W T, Keverne E B. The evolution of pheromonal communication. Behavioural Brain Res, 2009, 200: 239–247
Rouquier S, Blanher A, Giorgi D. The olfactory receptor gene repertoire in primates and mouse: Evidence for reduction of the functional fraction in primates. Proc Natl Acad Sci USA, 2000, 97: 2870–2874
Hunt D M, Dulai K S, Cowing J A, et al. Molecular evolution of trichromacy. Vision Res, 1998, 38: 3299–3306
Nathans J. The evolution and physiology of human color vision: Insights from molecular genetic studies of visual pigments. Neuron, 1999, 24: 299–312
Hudson R, Distel H. Pheromonal release of suckling in rabbits does not depend on the vomeronasal organ. Physiol Behav, 1986, 37: 123–128
Dorries K M, Adkins-Regan E, Halpern B P. Sensitivity and behavioral responses to the pheromone androstenone are not mediated by the vomeronasal organ in domestic pigs. Brain Behav Evol, 1997, 49: 53–62
Sam M, Vora S, Malnic B, et al. Neuropharmacology. Odorants may arouse instinctive behaviours. Nature, 2001, 412: 142
Trinh K, Storm D R. Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium. Nat Neurosci, 2003, 6: 519–525
Author information
Authors and Affiliations
Corresponding authors
Additional information
These authors contributed equally to this work
About this article
Cite this article
Yang, H., Meng, X., Yu, L. et al. Advances in research of mammalian vomeronasal pheromone perception and genetic components unique to vomeronasal signal transduction pathway. Chin. Sci. Bull. 55, 2473–2478 (2010). https://doi.org/10.1007/s11434-010-3141-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-010-3141-4