Abstract
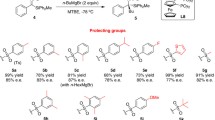
Highly diastereoselective addition of lithium enolate of γ-substituted α-diazoacetoacetate to chiral N-sulfinyl imines has been developed. The addition products were further subjected to photo-induced Wolff rearrangement or Rh(II)-catalyzed intramolecular N-H insertion to afford chiral 4,5-disubstituted 2-oxo and 3-oxo pyrrolidines, respectively.
Similar content being viewed by others
References
Daly J W, Spande T F, Garraffo H M. Alkaloids from Amphibian Skin:⊊sp. A tabulation of over eight-hundred compounds. J Nat Prod, 2005, 68: 1556–1575
O H D. Pyrrole, pyrrolidine, pyridine, piperidine and tropane alkaloids. Nat Prod Rep, 2000, 17: 435–446
Corey E J, Li W D Z. Total synthesis and biological activity of lactacystin, omuralide and analogs. Chem Pharm Bull, 1999, 47: 1–10
Huang P Q. New Methods for the Asymmetric Synthesis of Nitrogen Heterocycles. Research Signpost, Trivandrum India, 2005
Smith M B. Science of Synthesis. Georg Thieme Verlag: Stuttgart Germany, 2005
Renaud P, Giraud L. 1-amino- and 1-amidoalkyl radicals: Generation and stereoselective reactions. Synthesis, 1996, 913–926
Fallis A G, Brinza I M. Free radical cyclizations involving nitrogen. Tetrahedron, 1997, 53: 17543–17594
Ley S V, Cox L R, Meek G. (π-Allyl)tricarbonyliron lactone complexes in organic synthesis: A useful and conceptually unusual route to lactones and lactams. Chem Rev, 1996, 96: 423–442
Moyer M P, Feldman P L, Rapoport H. Intramolecular nitrogenhydrogen, oxygen-hydrogen and sulfur-hydrogen insertion reactions. Synthesis of heterocycles from α-diazo β-keto esters. J Org Chem, 1985, 50: 5223–5230
Clark J S, Hodgson P B. Intramolecular generation and rearrangement of ammonium ylides from copper carbenoids: a general method for the synthesis of cyclic amines. J Chem Soc Chem Commun, 1994, 23: 2701–2702
Wang J, Hou Y, Wu P. Intramolecular N-H insertion of α-diazocarbonyls catalyzed by Cu(acac)2: An efficient route to derivatives of 3-oxoazetidines, 3-oxopyrrolidines and 3-oxopiperidines. J Chem Soc Perkin Trans 1, 1999, 2277–2280
Yang H, Jurkauskas V, Mackintosh N, et al. Trifluoroacetic acid-mediated intramolecular formal N-H insertion reactions with amino-α-diazoketones: A facile and efficient synthesis of optically pure pyrrolidinones and piperidinones. Can J Chem, 2000, 78: 800–808
Davis F A, Fang T, Goswami R. Asymmetric synthesis of substituted prolines from δ-amino β-ketoesters. Methyl (2S, 5R)-(+)-5-phenylpyrrolidine-2-carboxylate. Org Lett, 2002, 4: 1599–1602
Berlin S, Ericsson C, Engman L. Radical carbonylation/reductive cyclization for the construction of tetrahydrofuran-3-ones and pyrrolidin-3-ones. J Org Chem, 2003, 68: 8386–8396
Davis F A, Deng J. Asymmetric total synthesis of (-)-agelastatin a using sulfinimine (N-sulfinyl imine) derived methodologies. Org Lett, 2005, 7: 621–623
Davis F A, Zhang J, Li Y, et al. Asymmetric synthesis of 2,4,5- trisubstituted piperidines from sulfinimine-derived δ-amino β-ketoesters. Formal synthesis of pseudodistomin B triacetate. J Org Chem, 2005, 70: 5413–5419
Davis F A, Yang B. Asymmetric synthesis of α-substituted β-amino ketones from sulfinimines (N-sulfinyl imines). Synthesis of the indolizidine alkaloid (−)-223A. J Am Chem Soc, 2005, 127: 8398–8407
Davis F A, Xu H, Wu Y, et al. Asymmetric synthesis of polyfunctionalized pyrrolidines from sulfinimine-derived pyrrolidine 2-phosphonates. Synthesis of pyrrolidine 225C. Org Lett, 2006, 8: 2273–2276
Davis F A, Melamed J Y, Sharik S S. Total synthesis of (−)-normalindine via addition of metalated 4-methyl-3-cyanopyridine to an enantiopure sulfinimine. J Org Chem, 2006, 71: 8761–8766
Davis F A, Santhanaraman M. Asymmetric synthesis of (−)-nupharamine and (−)-(5S,8R,9S)-5-(3-furyl)-8-methyl octahydroindolizidine from β-amino ketones and the intramolecular mannich reaction. J Org Chem, 2006, 71: 4222–4226
Davis F A. Adventures in sulfur-nitrogen chemistry. J Org Chem, 2006, 71: 8993–9003
Davis F A, Song M, Augustine A. Asymmetric synthesis of trans-2,5-disubstituted pyrrolidines from enantiopure homoallylic amines. Synthesis of pyrrolidine (−)-197B. J Org Chem, 2006, 71: 2779–2786
Davis F A, Ramachandar T, Chai J, et al. Asymmetric synthesis of α-amino aldehydes from sulfinimine (N-sulfinyl imine)-derived α-amino 1,3-dithianes. Formal synthesis of (−)-2,3-trans-3,4-cis-dihydroxyproline. Tetrahedron Lett, 2006, 47: 2743–2746
Davis F A, Xu H, Zhang J. Asymmetric synthesis of ring functionalized trans-2,6-disubstituted piperidines from N-sulfinyl δ-amino β-keto phosphonates. Total synthesis of (−)-myrtine. J Org Chem, 2007, 72: 2046–2052
Davis F A, Zhang Y, Li D. Sulfinimine-derived 2,3-diamino esters in the asymmetric synthesis of piperidine (2S,3S)-(+)-CP-99,994. Tetrahedron Lett, 2007, 48: 7838–7840
Davis F A, Ramachandar T. α-Amino 1,3-dithioketal mediated asymmetric synthesis of piperidines (L-733,060) and tetrahydrofuran glycines. Tetrahedron Lett, 2008, 49: 870–872
Weix D J, Shi Y, Ellman J A. Diastereoselective and enantioselective Rh(I)-catalyzed additions of arylboronic acids to N-tert-butanesulfinyl and N-diphenylphosphinoyl aldimines. J Am Chem Soc, 2005, 127: 1092–1093
Peltier H M, Ellman J A. N-sulfinyl metalloenamine conjugate addi tions: Asymmetric synthesis of piperidines. J Org Chem, 2005, 70: 7342–7345
Beenen M A, Weix D J, Ellman J A. Asymmetric synthesis of protected arylglycines by rhodium-catalyzed addition of arylboronic acids to N-tert-butanesulfinyl imino esters. J Am Chem Soc, 2006, 128: 6304–6305
Patterson A W, Ellman J A. Asymmetric synthesis of α,α-dibranched propargylamines by acetylide additions to N-tert-butanesulfinyl ketimines. J Org Chem, 2006, 71: 7110–7112
Dong C, Mo F, Wang J. Highly diastereoselective addition of the lithium enolate of α-diazoacetoacetate to N-sulfinyl imines: Enantioselective synthesis of 2-oxo and 3-oxo pyrrolidines. J Org Chem, 2008, 73: 1971–1974
Davis F A, Zhang Y, Andemichael Y, et al. Improved synthesis of enantiopure sulfinimines (thiooxime s-oxides) from p-toluenesulfinamide and aldehydes and ketones. J Org Chem, 1999, 64: 1403–1406
García R J, Alemán J, Belén C M, et al. A general method for the preparation of N-sulfonyl aldimines and ketimines. Org Lett, 2005, 7: 179–182
Green G M, Peet N P, Metz W A. Polystyrene-supported benzenesulfonyl azide: A diazo transfer reagent that is both efficient and safe. J Org Chem, 2001, 66: 2509–2511
Davies H M L, Cantrell W R Jr, Romines K R, et al. Synthesis of furans via rhodium(II) acetate-catalyzed reaction of acetylenes with α-diazocarbonyls: Ethyl 2-methyl-5-phenyl-3-furancarboxylate. Org Synth, 1992, 70: 93–100
Holmquist C R, Roskamp E J. A selective method for the direct conversion of aldehydes into β-keto esters with ethyl diazoacetate catalyzed by tin(II) chloride. J Org Chem, 1989, 54: 3258–3260
Davies F A, Zhang Y, Qiu H. Asymmetric synthesis of anti- and syn-2,3-diamino esters using sulfinimines. Water concentration effects. Org Lett, 2007, 9: 833–836
Wolfgang K. 100 Years of the Wolff Rearrangement. Eur J Org Chem, 2002, 2193-2256
Wang J, Hou Y. Wolff rearrangement of diazo ketones derived from N-p-tolylsulfonyl-protected α- and β-amino acids. J Chem Soc Perkin Trans 1, 1998, 1919–1924
Wang J, Hou Y, Wu P, et al. Stereoselective synthesis of enantiomerically pure 4,5-disubstituted pyrrolidinones from β-amino esters. Tetrahedron: Asymmetry, 1999, 10: 4553–4561
Lee D J, Kim K, Park Y J. Novel synthesis of 5,6-dihydro-4H-thieno[3,2-b]pyrrol-5-ones via the rhodium(II)-mediated Wolff rearrangement of 3-(thieno-2-yl)-3-oxo-2-diazopropanoates. Org Lett, 2002, 4: 873–876
Tsuji J, Nisar M, Shimizu I. Facile palladium-catalyzed decarboxylation reaction of allylic β-keto esters. J Org Chem, 1985, 50: 3416–3417
Hayakawa Y, Kato H, Uchiyama M, et al. Allyloxycarbonyl group: A versatile blocking group for nucleotide synthesis. J Org Chem, 1986, 51: 2400–2402
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Mo, F., Li, F. & Wang, J. Diastereoselective addition of lithium enolate of γ-substituted α-diazoacetoacetate to N-sulfinyl imines. Chin. Sci. Bull. 55, 2847–2854 (2010). https://doi.org/10.1007/s11434-010-3140-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-010-3140-5