Abstract
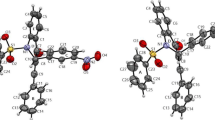
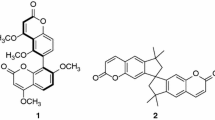
Three arylamide-bridged biscoumarin derivatives 1–3 have been designed and prepared. Compounds 1 and 2 are induced by the intramolecular N-H…O and N-H…F hydrogen bonding to possess a helical conformation, and 3 is induced to have an extended conformation. A comparison of their absorption and fluorescent spectra in a variety of solvents of a wide range of polarity with those of control compound 4 reveals that, for foldamers 1 and 2, the intramolecular hydrogen bonding and the helical conformations exist in most solvents, but do not exist or are very weak in DMF and DMSO.
Similar content being viewed by others
References
Li Z T, Hou J L, Li C. Peptide mimics by linear arylamides: A structural and functional diversity test. Acc Chem Res, 2008, 41: 1343–1353
Gong B. Hollow crescents, helices, and macrocycles from enforced folding and folding-assisted macrocyclization. Acc Chem Res, 2008, 41: 1376–1386
Li Z T, Hou J L, Li C, et al. Shape-persistent aromatic amide oligomers: new tools for supramolecular chemistry. Chem Asian J, 2006, 1: 766–778
Huc I. Aromatic oligoamide foldamers. Eur J Org Chem, 2004, 17–29
Hamuro Y, Geib S J, Hamilton D. Novel folding patterns in a family of oligoanthranilamides: Non-peptide oligomers that form extended helical secondary structures. J Am Chem Soc, 1997, 119: 10587–10593
Zhu J, Parra R D, Gong B, et al. A new class of folding oligomers: Crescent oligoamides. J Am Chem Soc, 2000, 122: 4219–4220
Jiang H, Léger J M, Huc I. Aromatic δ-peptides. J Am Chem Soc, 2003, 125: 3448–3449
Hou J L, Shao X B, Li Z T, et al. Hydrogen bonded oligohydrazide foldamers and their recognition for saccharides. J Am Chem Soc, 2004, 126: 12386–12394
Hu Z Q, Hu H Y, Chen C F. Phenanthroline dicarboxamide-based helical foldamers: Stable helical structures in methanol. J Org Chem, 2006, 71: 1131–1138
Gan Q, Huc I, Jiang H, et al. Quadruple and double helices of 8-fluoroquinoline oligoamides. Angew Chem Int Ed, 2008, 47: 1715–1718
Xu X N, Lin J B, Li Z T, et al. Hydrogen bonding-mediated dynamic covalent synthesis of imine-based capsules and formation of pseudo [3]rotaxanes. Chem Eur J, 2009, 15: 5763–5774
Cai W, Wang G T, Li Z T, et al. Vesicles and organogels from foldamers: A solvent-modulated self-assembling process. J Am Chem Soc, 2008, 130: 6936–6937
Parra R D, Zeng H, Gong B, et al. Stable three-center hydrogen bonding in a partially rigidified structure. Chem Eur J, 2001, 7: 4352–4357
Yu X, Scheller D, Rademacher O, et al. Selectivity in the photodimerization of 6-alkylcoumarins. J Org Chem, 2003, 68: 7386–7399
Gompel J V, Schuster G B. Chemiluminescence of organic peroxides: Intramolecular electron-exchange luminescence from a secondary perester. J Org Chem, 1987, 52: 1465–1468
Zhu Y Y, Wu J, Li Z T, et al. F…H-N and MeO…H-N Hydrogenbonding in the solid states of aromatic amides and hydrazides. A comparison study. Cryst Growth Des, 2007, 7: 1490–1496
Li C, Ren S F, Li Z T, et al. F…H-N hydrogen bonding driven foldamers: Efficient receptors for dialkylammonium ions. Angew Chem Int Ed, 2005, 44: 5725–5729
Wu J, Fang F, Li Z T, et al. Dynamic [2]catenanes based on hydrogen bonding-mediated bis-zinc oorphyrin foldamer tweezer: A case study. J Org Chem, 2007, 72: 2897–2905
Jones G II, Jackson W R, Choi C Y, et al. Solvent effects on emission yield and lifetime for coumarin laser dyes requirements for a rotatory decay mechanism. J Phys Chem, 1985, 89: 294–300
Anslyn E V, Dougherty D A. Modern Physical Organic Chemistry. Sausalito: University Science Books, 2005. 147
Lin C H, Tung Y C, Ruokolainen J, et al. Poly[2,7-(9,9-dihexylfluorene)]-block-poly(2-vinylpyridine) rod-coil and coil-rod-coil block copolymers: Synthesis, morphology and photophysical properties in methanol/THF mixed solvents. Macromolecules, 2008, 41: 8759–8769
Balaban M C, Eichhoefer A, Buth G, et al. Programmed metalloporphyrins for self-assembly within light-harvesting stacks: (5,15-dicyano-10,20-bis(3,5-di-tert-butylphenyl)porphyrinato)zinc (II) and its push-pull 15-N,N-dialkylamino-5-cyano congeners obtained by a facile direct amination. J Phys Chem B, 2008, 112: 5512–5521
Jones G II, Jackson W R, Halpern A M. Medium effects on fluorescence quantum yields and lifetimes for coumarin laser dyes. Chem Phys Lett, 1980, 72: 391–395
Brinas R P, Troxler T, Hochstrasser R M, et al. Phosphorescent oxygen sensor with dendritic protection and two- photon absorbing antenna. J Am Chem Soc, 2005, 127: 11851–11862
Yamaguchi A, Amino Y, Shima K, et al. Local environments of coumarin dyes within mesostructured silica-surfactant nanocomposites. J Phys Chem B, 2006, 110: 3910–3916
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Lu, Z., Zhu, Y., Lin, J. et al. Hydrogen bonded foldamer-bridged biscoumarins: A UV-Vis absorption and fluorescent study of the solvent effect. Chin. Sci. Bull. 55, 2870–2878 (2010). https://doi.org/10.1007/s11434-010-3132-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-010-3132-5