Abstract
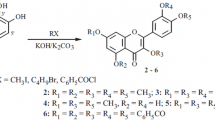
4-Hydroxycinnamic acid derivatives, including ferulic acid (FA) (1), sinapic acid (SA) (2) and caffeic acid (CA) (3), are widely distributed in the plant kingdom, and undergo oxidative cross-coupling leading to the corresponding dehydrodimers, trimers and even higher oligomers in plants. In order to evaluate the antioxidative ability of these 4-hydroxycinnamic acid derivatives and their oligomers, we synthesized 8-8′-bis-lactone-dimers (8-8′-DiFA (4), 8-8′-DiSA (5) and 8-8′-DiCA (6)), as well as a new trimer (7), by the reaction of the corresponding monomers (1–3) with Ag2O in methanol, and assayed their free radical-scavenging activity by the reaction with 2,2-diphenyl-1-picrylhydrazyl radical (DPPH·). It was found that CA (3) and 8-8′-DiCA (6) bearing o-dihydroxyl groups exhibited significantly higher radical-scavenging activity than those bearing no such groups, and oxidative coupling of CA (3) resulted in remarkable enhancement in the activity.
Similar content being viewed by others
References
Finkel T, Holbrook N J. Oxidants, oxidative stress and the biology of ageing. Nature, 2000, 408: 239–247
Perwez H S, Hofseth L J, Harris C C. Radical cause of cancer. Nat Rev Cancer, 2003, 3: 276–285
Cooke M S, Evans M D, Dizdaroglu M, et al. Oxidative DNA damage: Mechanism, mutation, and disease. FASEB J, 2003, 17: 1195–1214
Surh Y J. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer, 2003, 3: 768–780
Brash D E, Harve P A. New careers for antioxidants. Proc Natl Acad Sci USA, 2002, 99: 13969–13971
Rice-Evans C A, Diplock A T. Current status of antioxidant therapy. Free Radic Biol Med, 1993, 15: 77–96
Zhou B, Liu Z L. Bioantioxidants: From chemistry to biology. Pure Appl Chem, 2005, 77: 1887–1903
Robbins R J. Phenolic acids in foods: An overview of analytical methodology. J Agric Food Chem, 2003, 51: 2866–2887
Castelluccio C, Paganga G, Melikian N, et al. Antioxidant potential of intermediates in phenylpropanoid metabolism in higher plants. FEBS Lett, 1995, 368: 188–192
Nardini M, D’Aquino M, Tomassi G, et al. Inhibition of human low-density lipoprotein oxidation by caffeic acid and other hydroxycinnamic acid derivatives. Free Radic Biol Med, 1995, 19: 541–552
Firuzi O, Giansanti L, Vento R, et al. Hypochlorite scavenging activity of hydroxycinnamic acids evaluated by a rapid microplate method based on the measurement of chloramines. J Pharm Pharmacol, 2003, 55: 1021–1027
Pannala A, Razaq R, Halliwell B, et al. Inhibition of peroxynitrite dependent tyrosine nitration by hydroxycinnamates: Nitration or electron donation? Free Radic Biol Med, 1998, 24: 594–606
Chen J H, Ho C T. Antioxidant activities of caffeic acid and its related hydroxycinnamic acid compounds. J Agric Food Chem, 1997, 45: 2374–2378
Zhou B, Miao Q, Yang L, et al. Antioxidative effects of flavonols and their glycosides against the free-radical-induced peroxidation of linoleic acid in solution and in micelles. Chem Eur J, 2005, 11: 680–691
Zhou B, Wu L M, Yang L, et al. Evidence for α-tocopherol regeneration reaction of green tea polyphenols in SDS micelles. Free Radic Biol Med, 2005, 38: 78–84
Chen W F, Deng S L, Zhou B, et al. Curcumin and its analogues as potent inhibitors of low density lipoprotein oxidation: H-atom abstraction from the phenolic groups and possible involvement of the 4-hydroxy-3-methoxyphenyl groups. Free Radic Biol Med, 2006, 40: 526–535
Zheng L F, Wei Q Y, Cai Y J, et al. DNA damage induced by resveratrol and its synthetic analogues in the presence of Cu (II) ions: Mechanism and structure-activity relationship. Free Radic Biol Med, 2006, 41: 1807–1816
Qian Y P, Cai Y J, Fan G J, et al. Antioxidant-based lead discovery for cancer chemoprevention: The case of resveratrol. J Med Chem, 2009, 52: 1963–1974
Shang Y J, Qian Y P, Liu X D, et al. Radical-scavenging activity and mechanism of resveratrol-oriented analogues: Influence of the solvent, radical and substitution. J Org Chem, 2009, 74: 5025–5031
Fang J G, Zhou B. Structure-activity relationship and mechanism of the tocopherol-regenerating activity of resveratrol and its analogues. J Agric Food Chem, 2008, 56: 11458–11463
Cheng J C, Dai F, Zhou B, et al. Antioxidative activity of hydroxycinnamic acid derivatives in human low density lipoprotein: Mechanism and structure-activity relationship. Food Chem, 2007, 104: 132–139
Bunzel M, Ralph J, Funk C, et al. Structural elucidation of new ferulic acid-containing phenolic dimers and trimers isolated from maize bran. Tetrahedron Lett, 2005, 46: 5845–5850
Funk C, Ralph J, Steinhart H, et al. Isolation and structural characterization of 8-O-4/8-O-4- and 8-8/8-O-4-coupled dehydrotriferulic acids from maize bran. Phytochemistry, 2005, 66: 363–371
Bunzel M, Ralph J, Bruening P, et al. Structural identification of dehydrotriferulic and dehydrotetraferulic acids isolated from insoluble maize bran fiber. J Agric Food Chem, 2006, 54: 6409–6418
Tanaka T, Nishimura A, Kouno I, et al. Isolation and characterization of yunnaneic acids A-D, four novel caffeic acid metabolites from Salvia yunnanensis. J Nat Prod, 1996, 59: 843–849
Tanaka T, Nishimura A, Kouno I, et al. Four new caffeic acid metabolites, yunnaneic acids E-H, from Salvia yunnanensis. Chem Pharm Bull, 1997, 45: 1596–1600
Cano A, Arnao M B, Williamson G, et al. Superoxide scavenging by polyphenols: Effect of conjugation and dimerization. Redox Rep, 2002, 7: 379–383
Cartwright N J, Haworth R D. Constituents of natural phenolic resins. XIX. Oxidation of ferulic acid. J Chem Soc, 1944, 535–537
Kumada Y, Takeuchi T, Umezawa H. Characterization of the dehydrodicaffeic acid dilactone-forming enzyme and the enzymic and chemical synthesis of this mushroom product. Agric Biol Chem, 1977, 41: 877–885
Sako M, Hosokawa H, Ito T, et al. Regioselective oxidative coupling of 4-hydroxystilbenes: Synthesis of resveratrol and ɛ-viniferin (E)-dehydrodimers. J Org Chem, 2004, 69: 2598–2600
West K F, Moore H W. An unusual oxidative dimerization of 2-(vinyloxy)phenols. J Org Chem, 1984, 49: 2809–2812
Liu H L, Kong L Y, Takaya Y, et al. Biotransformation of ferulic acid into two new dihydrotrimers by Momordica charantia peroxidase. Chem Pharm Bull, 2005, 53: 816–819
Ward G, Hadar Y, Bilkis I, et al. Initial steps of ferulic acid polymerization by lignin peroxidase. J Biol Chem, 2001, 276: 18734–18741
Taylor E C, Andrade J G, Rall G J H, et al. Thallium in organic synthesis. 60. 2,6-Diaryl-3,7-dioxabicyclo[3.3.0]octane-4,8-dione lignans by oxidative dimerization of 4-alkoxycinnamic acids with thallium (III) trifluoroacetate or cobalt(III) trifluoride. J Org Chem, 1981, 46: 3078–3081
Tazaki H, Taguchi D, Hayashida T, et al. Stable isotope-labeling studies on the oxidative coupling of caffeic acid via o-quinone. Biosci Biotechnol Biochem, 2001, 65: 2613–2621
Goupy P, Dufour C, Loonis M, et al. Quantitative kinetic analysis of hydrogen transfer reactions from dietary polyphenols to the DPPH radical. J Agric Food Chem, 2003, 51: 615–622
Foti M, Ruberto G. Kinetic solvent effects on phenolic antioxidant determined by spectrophotometric measurements. J Agric Food Chem, 2001, 49: 342–348
Wright J S, Johnson E R, Dilabio G A. Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidant. J Amer Chem Soc, 2001, 123: 1173–1183
Author information
Authors and Affiliations
Corresponding authors
About this article
Cite this article
Jin, X., Yang, R., Shang, Y. et al. Oxidative coupling of cinnamic acid derivatives and their radical-scavenging activities. Chin. Sci. Bull. 55, 2885–2890 (2010). https://doi.org/10.1007/s11434-010-3064-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-010-3064-0