Abstract
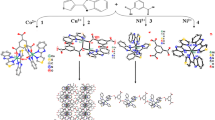
The hydrothermal synthesis and crystal structures of four coordination polymers, namely, 2D [Zn(μ3-ta)(pytaH)] n (1), 2D [Zn(μ3-pyta)Cl] n (2), 1D [Cd(μ-pyta)2(H2O)] n (3), and 3D [Cd(μ3-pyta)(μ-Cl)] n (4) (pyta = (4-pyridylthio)acetate, ta = thioglycolate), are reported. They are based on (4-pyridylthio)acetate and its derived ligand. The ta2− ligand present in 1 was generated from an in situ C(sp2)-S bond cleavage of the pyta ligand. In these compounds, versatile intermolecular interactions, such as close S…S interactions and strong (O-H…O/N/S) or weak (C-H…O/S, C-H…Cl) hydrogen bonding interactions, play an important role in the formation of three-dimensional supramolecular networks in the solid state.
Similar content being viewed by others
References
Hoskins B F, Robson R. Design and construction of a new class of scaffolding-like materials comprising infinite polymeric frameworks of 3D-linked molecular rods. A reappraisal of the Zn(CN)2 and Cd(CN)2 structures and the synthesis and structure of the diamond-related frameworks [N(CH3)4][CuIZnII(CN)4] and CuI[4,4′,4″, 4‴-tetracyanotetraphenylmethane] BF4·xC6H5NO2. J Am Chem Soc, 1990, 112: 1546
Batten S R, Robson R. Interpenetrating nets: Ordered, periodic entanglement. Angew Chem Int Ed, 1998, 37: 1460
Feng S H, Xu R R. New materials in hydrothermal synthesis. Acc Chem Res, 2001, 34: 239
Kitagawa S, Kitaura R, Noro S. Functional porous coordination polymers. Angew Chem Int Ed, 2004, 43: 2334
Kitagawa S, Uemura K. Dynamic porous properties of coordination polymers inspired by hydrogen bonds. Chem Soc Rev, 2005, 34: 109
Kamruddin S, Roy A. Synthesis and characterization of Cr(III), Mn(II), Fe(III), Co(II), Ni(II) and Cu(II) complexes of 4-pyridyl thioacetic acid and 2-pyrimidyl thioacetic acid. Indian J Chem A, 2001, 40: 211
Kondo M, Miyazawa M, Irie Y, et al. A new Zn(II) coordination polymer with 4-pyridyl-thioacetate: Assemblies of homo-chiral helices with sulfide sites. Chem Commun, 2002, 2156
Du M, Zhao X J, Wang Y. Crystal engineering of a versatile building block toward the design of novel inorganic-organic coordination architectures. Dalton Trans, 2004, 2065
Zhang X M, Fang R Q, Wu H S. A twelve-connected Cu6S4 cluster-based coordination polymer. J Am Chem Soc, 2005, 127: 7670
Tong M L, Wu Y M, Ru J, et al. Pseudo-polyrotaxane and beta-sheet layer-based three-dimensional coordination polymers constructed with silver salts and flexible pyridyl-type ligands. Inorg Chem, 2002, 41: 4846
Tong M L, Chen X M, Batten S R. A new self-penetrating uniform net, (8,4) (or 8(6)), containing planar four-coordinate nodes. J Am Chem Soc, 2003, 125: 16170
Hu S, Chen J C, Tong M L, et al. Cu2+-mediated dehydrogenative coupling and hydroxylation of an N-heterocyclic ligand: From gene-ration of a new tetratopic ligand to the designed assembly of three-dimensional copper(I) coordination polymers. Angew Chem Int Ed, 2005, 44: 5471
Wang J, Zheng L L, Li C J, et al. Coexistence of planar and chair-shaped cyclic water hexamers in a unique cyclohexanehexacarboxylate-bridged metal-organic framework. Cryst Growth Des, 2006, 6: 357
Wang J, Ou Y C, Shen Y, et al. Coordination chemistry of cyclohexane-1,2,4,5-tetracarboxylate(H4L). Synthesis, structure and magnetic properties of metal-organic frameworks with conformation-flexible H4L ligand. Cryst Growth Des, 2009, 9: 2442
Wang J, Lin Z J, Ou Y C, et al. Coordination chemistry of conformation-flexible 1,2,3,4,5,6-cyclohexanehexacarboxylate: Trapping various conformations in metal-organic frameworks. Chem Eur J, 2008, 14: 7218
Chen X M, Tong M L. Solvothermal in situ metal/ligand reactions: A new bridge between coordination chemistry and organic synthetic chemistry. Acc Chem Res, 2007, 40: 162
Sheldrick G M. SADABS 2.05. Göttingen: University of Göttingen, 2000
SHELXTL 6.10. Bruker Analytical Instrumentation. Madison, Wisconsin, USA, 2000
Jeannin S, Jeannin Y, Lavigne G. Étude cristallographique du complexe 1-1 du fer(II) et de l’acide thioglycolique. J Organomet Chem, 1972, 40: 175
Wenzel B, Lönnecke P, Stender M, et al. Early/late heterobimetallics: Unusual tetranuclear Zr/Ni and octanuclear Zr/Pd complexes with bridging bifunctional sulfidoacetato ligands. J Chem Soc, Dalton Trans, 2002, 478
Bikker P J M W L. Copper thiolate cluster compounds. X-ray structure and properties of pentathallium(I)-μ8-chloro-dodecakis(α-mercaptoisobutyrato)octacuprate(I)hexacuprate(II) hydrate, Tl5[CuII 6CuI 8(SC(CH3)2COO)12Cl]·∼12H2O. Inorg Chem, 1979, 18: 3502
Etter M C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc Chem Res, 1990, 23: 120
Pauling L. The Nature of the Chemical Bond. 3rd ed. Ithaca, NY: Cornell University Press, 1973
Tzeng B C, Yeh H T. Self-assembly of a Cd2+ compound with 4-pyridylthioacetic acid: Structural and luminescence properties. Z Naturforsch B, 2004, 59: 1320
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supported by the National Natural Science Foundation of China (Grant No. 20525102), Specialized Research Foundation for the Doctoral Program of Higher Education (Grant No. 20060558081), “Hundred Talents” Foundation of Sun Yat-Sen University, Doctoral Foudation of Ministry of Education of China for New Scholar (Grant No. 200805581015), and Natural Science Foundation of Guangdong Province (Grant No. 8451027501001515)
About this article
Cite this article
Zhang, Y., Wang, J., Zheng, L. et al. Structural diversity and reactivity of d10 metal-(4-pyridylthio)acetate system. Chin. Sci. Bull. 54, 4277–4284 (2009). https://doi.org/10.1007/s11434-009-0673-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-009-0673-6