Abstract
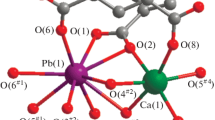
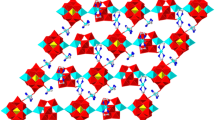
A new 3-D porous Gd-Cu heterometallic polymer [Gd2Cu3(bpy)2(ip)6]·6H2O (1) (bpy = 2,2′-bipyridine, H2ip = isophthalic acid) has been hydrothermally synthesized and structurally characterized. Compound 1 crystallizes in the triclinic space group P \( \bar 1 \) and displays a 3-D non-interpenetrated α-Po network with 1-D channels filled transversely by the hexa-nuclear chain-like (H2O)6. The EPR and thermal stability of 1 were investigated.
Similar content being viewed by others
References
Xia J, Zhao B, Wang H S, et al. Two- and three-dimensional lanthanide complexes: Synthesis, crystal structures, and properties. Inorg Chem, 2007, 46: 3450–3458
Cheng J W, Zheng S T, Yang G Y. Diversity of crystal structure with different lanthanide ions involving in situ oxidation-hydrolysis reaction. Dalton Trans, 2007, 36: 4059–4066
Zhu W H, Wang Z M, Gao S. Two 3D porous lanthanide-fumarate-oxalate frameworks exhibiting framework dynamics and luminescent change upon reversible de- and rehydration. Inorg Chem, 2007, 46: 1337–1342
Kitagawa S, Kitaura R, Noro S. Functional porous coordination polymers. Angew Chem Int Ed, 2004, 43: 2334–2375
Liu G X, Zhu K, Chen H, et al. Two zinc(II) supramolecular isomers of square grid networks formed by two flexible ligands: Syntheses, structures and nonlinear optical properties. CrystEngComm, 2008, 10: 1527–1530
Ren P, Shi W, Cheng P. Synthesis and characterization of three-dimensional 3d–3d and 3d–4f heterometallic coordination polymers with high thermal stability. Cryst Growth Des, 2008, 8: 1097–1099
Gao H L, Yi L, Ding B, et al. First 3D Pr(III)-Ni(II)-Na(I) polymer and a 3D Pr(III) open network based on pyridine-2,4,6-tricarboxylic acid. Inorg Chem, 2006, 45: 481–483
Gu X J, Xue D F. 3D coordination framework [Ln4(μ3-OH)2Cu6I5-. (IN)8 (OAc)3] (IN=isonicotinate): Employing 2D layers of lanthanide wheel clusters and 1D chains of copper halide clusters. Inorg Chem, 2007, 46: 5349–5353
Murugesu M, Clérac R, Anson C E, et al. Structure and magnetic properties of a giant Cu44 II aggregate which packs with a zeotypic superstructure. Inorg Chem, 2004, 43: 7269–7271
Zheng N F, Bu X H, Lu H W, et al. Crystalline superlattices from single-sized quantum dots. J Am Chem Soc, 2005, 127: 11963–11965
Zhao B, Cheng P, Dai Y, et al. A nanotubular 3D coordination polymer based on a 3d–4f heterometallic assembly. Angew Chem Int Ed, 2003, 42: 934–936
Cheng J W, Zhang J, Yang G Y. Lanthanide-transition-metal sandwich framework comprising {Cu3} cluster pillars and layered networks of {Er36} wheels. Angew Chem Int Ed, 2006, 45: 73–77
Cheng J W, Zheng S T, Yang G Y. Linking two distinct layered networks of nanosized {Ln18} and {Cu24} wheels through isonicotinate ligands. Chem-Eur J, 2008, 14: 88–97
Zhang M B, Zhang J, Yang G Y. A 3D coordination framework based on linkages of nanosized hydroxo lanthanide clusters and copper centers by isonicotinate ligands. Angew Chem Int Ed, 2005, 44: 1385–1388
Cheng J W, Zheng S T, Yang G Y. {LnIII[μ5-K2,K1,K1,K1,K1-1,2-(CO2)2C6H4][isonicotine][H2O]}2CuI·X(Ln= Eu, Sm, Nd; X =ClO4 −, Cl−): A new pillared-layer approach to heterobimetallic 3d–4f 3D-network solids. Inorg Chem, 2007, 46: 10534–10538
Cheng J W, Zheng S T, Yang G Y. Incorporating distinct metal clusters to construct diversity of 3D pillared-layer lanthanide-transition-metal frameworks. Inorg Chem, 2008, 47: 4930–4935
Sheldrick G M. SHELXS97. Program for Crystal Structure Solution. Göttingen, Germany: University of Göttingen, 1997
Sheldrick G M. SHELXL97. Program for Crystal Structure Refinement. Göttingen, Germany: University of Göttingen, 1997
He F, Tong M L, Chen X M, et al. Synthesis, structures, and magnetic properties of heteronuclear Cu(II)-Ln(III) (Ln = La, Gd, or Tb) complexes. Inorg Chem, 2005, 44: 8285–8292
Niu S Y, Jin J, Jin X X, et al. Synthesis, structure and characterization of Gd(III) dimer bridged by tetra benzoates. Solid State Sci, 2002, 4: 1103–1106
Zhang M L, Li D S, Fu F, et al. A novel 4·82 CdII network constructed from helical motif: Incorporating alternate left- and right-hand helical water chains. Inorg Chem Comm, 2008, 11: 958–960
Martin D P, Montney M R, Supkowski R M, et al. Cadmium glutarate coordination polymers containing hydrogen-bonding capable tethering organodiimines: From double interpenetration to supra-molecular cavities containing an unprecedented water tape morphology. Cryst Growth Des, 2008, 8: 3091–3097
Zhao B, Cheng P, Chen X Y. Design and synthesis of 3d–4f metal-based zeolite-type materials with a 3D nanotubular structure encapsulated “water” pipe. J Am Chem Soc, 2004, 126: 3012–3013
Li Y, Jiang L, Feng X L, et al. Anion dependent water clusters encapsulated inside a cryptand cavity. Cryst Growth Des, 2008, 8: 3689–3694
Cheng J W, Zheng S T, Liu W, et al. An unusual eight-connected self-penetrating ilc net constructed by dinuclear lanthanide building units. CrystEngComm, 2008, 10: 765–769
Mir M H, Kitagawa S, Vittal J J. Two- and three-fold interpenetrated metal-organic frameworks from one-pot crystallization. Inorg Chem, 2008, 47: 7728–7733
Blatov V A, Carlucci L, Proserpio D M. Interpenetrating metal-organic and inorganic 3D networks: A computer-aided systematic investigation. part I. Analysis of the cambridge structural database. Cryst Eng Comm, 2004, 6: 377–395
Wang X L, Cao Q, Wang E B, et al. Metal nuclearity modulated four-, six-, and eight-connected entangled frameworks based on mono-, bi-, and trimetallic cores as nodes. Chem Eur J, 2006, 12: 2680–2691
Kesanli B, Cui Y, Smith M R, et al. Highly interpenetrated metal-organic frameworks for hydrogen storage. Angew Chem Int Ed, 2005, 44: 72–75
Ye B H, Ding B B, Weng Y Q, et al. Multidimensional networks constructed with isomeric benzenedicarboxylates and 2,2′-biimi-dazole based on mono-, bi-, and trinuclear units. Cryst Growth Des, 2005, 5: 801–806
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation for Distinguished Young Scholars of China (Grant No. 20725101), Major State Basic Research Development Program of China (Grant No. 2006CB932904), Natural Science Foundation of Fujian Province (Grant Nos. E0510030, 2008F3120), National Natural Science Fund of China (Grant Nos. 50872133, 20821061), and Knowledge Innovation Program of the CAS (Grant No. KJCX2.YW.H01)
About this article
Cite this article
Jia, X., Zhou, J., Zhao, J. et al. A new 3-D Gd-Cu heterometallic polymer [Gd2Cu3(bpy)2-(ip)6]·6H2O with a non-interpenetrated α-Po net. Chin. Sci. Bull. 54, 4272–4276 (2009). https://doi.org/10.1007/s11434-009-0549-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-009-0549-9