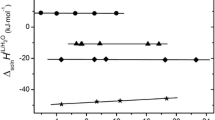
Abstract
On the basis of the theoretical linear solvation energy relationship (TLSER) suggested by Wilson et al. and the quantum chemical descriptors computed by AM1 Hamiltonian, a predicting model was developed to characterize the activity coefficients at infinite dilution γ ∞i of 34 organic solutes in ionic liquids (ILs) 1-butyl-3-methylimidazolium trifluoromethanesulfonate ([BMIM][CF3SO3]) and 1-propyl-2,3-dimethylimidazolium tetrafluoroborate ([PDMIM][BF4]) at 323.15 K. The results showed that the model had an good correlation and could successfully describe γ ∞i . In addition, correlation parameters are analyzed to understand the interactions that affect infinite dilution activity coefficients.
Similar content being viewed by others
References
Rogers R D, Seddon K R. Ionic liquids-solvents of the future. Science, 2003, 302: 792–793
Chiappe C, Pieraccini D. Ionic liquids: solvent properties and organic reactivity. J Phys Org Chem, 2005, 18: 275–297
Zhang S, Sun N, Zhang X, et al. Periodicity and map for discovery of new ionic liquids. Sci China Ser B, 2006, 49: 103–115
Ranke J, Stolte S, Stormann R, et al. Design of sustainable chemical products—the example of ionic liquids. Chem Rev, 2007, 107: 2183–2206
Wilson L Y, Famini G R. Using theoretical descriptors in quantitative structure-activity relationships: some toxicological indices. J Med Chem, 1991, 34: 1668–1674
Lowrey A H, Cramer C J, Urban J J, et al. Quantum chemical descriptors for linear solvation energy relationships. Computers & Chemistry, 1995, 19: 209–215
Katritzky A R, Fara D C, Yang H, et al. Quantitative measures of solvent polarity. Chem Rev, 2004, 104: 175–198
Huddleston J G, Willauer H D, Burney M T, et al. Comparison of an empirical and a theoretical linear solvation energy relationship applied to the characterization of solute distribution in a poly(ethylene) glycol-salt aqueous biphasic system. J Chem Inf Comput Sci, 2004, 44: 549–558
Sun C, Feng L. Approach to predict partitioning of organic compounds from air into airborne particulate. Chin Sci Bull, 2005, 50: 1696–1698
Long J J, Zhang M Q, Zhang X. QSPR research on the lipophilicity of monohydric alcohols based on theoretical linear solvation energy relationship (in Chinese). Computers and Applied Chemistry, 2005, 22: 883–890
Wang M H, Wu J S, Wang L S, et al. Activity coefficients at infinite dilution of alkanes, alkenes and alkyl benzenes in 1-propyl-2,3-dimethylimidazolium tetrafluoroborate using gas-liquid chromatography. J Chem Eng Data, 2007, 52: 1488–1491
Ge M L, Wang L S, Li M Y, et al. Activity coefficients at infinite dilution of alkanes, alkenes and alkyl benzenes in 1-butyl-3-methylimidazolium trifluoromethanesulfonate using gas-liquid chromatography. J Chem Eng Data, 2007, 52: 2257–2260
Ge M L, Wang L S. Activity coefficients at infinite dilution of polar solutes in 1-butyl-3-methylimidazolium trifluoromethanesulfonate using gas-liquid chromatography. J Chem Eng Data, 2008, 53: 846–849
Ge M L, Wu J S, Wang M H, et al. Activity coefficients at infinite dilution of polar solutes in 1-propyl-2,3-dimethylimidazolium tetrafluoroborate using gas-liquid chromatography. J Chem Eng Data, 2008, 53: 871–873
Kamlet M J, Doherty R, Taft R W, et al. Linear solvation energy relationships. 26. Some measures of relative self-association of alcohols and water. J Am Chem Soc, 1983, 105: 6741–6743
Dai J Y, Han S K, Wang L S. Prediction of partition properties of substituted benzaldehydes using different structural parameters (in Chinese). China Environmental Science, 2000, 20: 292–295
Mutelet F, Jaubert J-N, Rogalski M, et al. Thermodynamic properties of mixtures containing ionic liquids: Activity coefficients at infinite dilution of organic compounds in 1-propyl boronic acid-3-alkylimidazolium bromide and 1-propenyl-3-alkylimidazolium bromide using inverse gas chromatography. J Chem Eng Data, 2006, 51: 1274–1279
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Beijing Municipal Training Programme for the Excellent Talents (Grant No. 20081D0500500140)
About this article
Cite this article
Ge, M., Xiong, J. & Wang, L. Theoretical prediction for the infinite dilution activity coefficients of organic compounds in ionic liquids. Chin. Sci. Bull. 54, 2225–2229 (2009). https://doi.org/10.1007/s11434-009-0251-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-009-0251-y