Abstract
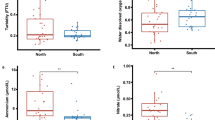
Coral reef bleaching is usually characterized by expulsion of symbiotic zooxanthellae, loss of zooxanthellae pigmentation, or both. We collected 128 samples comprising 39 species of 21 genera of reef-building corals from Luhuitou and Xiaodonghai in Sanya of Hainan Island and Daya Bay of Guangdong Province, respectively, and analyzed the symbiotic zooxanthellae population density. The results show that: (1) the symbiotic zooxanthella density varies from 0.67×106 to 8.48×106 cell/cm2, displaying significant interspecies variability, with branch corals usually having relatively less zooxanthellae (ranging from 0.67×106 to 2.47×106 cell/cm2) than massive species (from 1.0×106 to 8.48×106 cell/cm2); (2) corals inhabiting within 4 m water depth have higher levels of symbiotic zooxanthellae than those living at the bottom (∼7 m depth) of the reef area; (3) there is no discernable difference in the zooxanthellae density between corals from relatively high latitude Daya Bay (∼22°N) and those from relatively low latitude Sanya (∼18°N) at comparable sea surface temperatures (SST); (4) in partially-bleached corals, the density of zooxanthellae shows the following order: healthy-looking part> semi-bleached part > bleached part. Based on the above results, we suggest that (1) the zooxanthellae density difference between branching and massive coral species is the main cause that branching corals are more vulnerable to bleaching than massive corals. For example, symbiotic zooxanthellae levels are low in branching Acropora and Pocillopora corals and thus these corals are more susceptible to bleaching and mortality; (2) symbiotic zooxanthellae density can also be affected by environmental conditions, such as sediment loads, diving-related turbidity, and aquaculture-related nitrate and phosphate input, and their increase may reduce symbiotic zooxanthellae density in corals.
Similar content being viewed by others
References
Brown B E. Coral bleaching: causes and consequences. Coral Reefs, 1997, 16(Suppl1): 129–138
Li S, Yu K F. Recent development in coral reef bleaching research. Acta Ecol Sin (in Chinese), 2007, 27(5): 2059–2069
Wilkinson C. Status of Coral Reef of the World. Townsville: Australian Institute of Marine Science Press, 2004. 1–316
Yu K F, Zhao J X, Liu T S, et al. High-frequency winter cooling and reef coral mortality during the Holocene climatic optimum. Earth Planet Sci Lett, 2004, 224: 143–155
Goreau T F. Mass expulsion of zooxanthellae from Jamaican Reef communities after hurricane Flora. Science, 1964, 145: 383–386
Kushmaro A, Loya L, Fine M, et al. Bacterial infection and coral bleaching. Nature, 1996, 380: 396
Hoegh-Guldberg O. Climate, coral bleaching and the future of the world’s coral reefs. Mar Freshwater Res, 1999, 50: 839–866
Douglas A E. Coral bleaching—how and why? Mar Pollut Bull, 2003, 46(4): 385–392
Lough J M, 1997–98: Unprecedented thermal stress to coral reefs? Geophys Res Lett, 2000, 27(23): 3901–3904
Yu K F, Zhao J X, Shi Q, et al. U-series dating of dead Porites corals in the South China Sea: Evidence for episodic coral mortality over the past two centuries. Quaternary Geochronol, 2006, 1: 129–141
Arceo H, Quibilan M C, Alino P M, et al. Coral bleaching in Philippine reefs: Coincident evidences with mesoscale thermal anomalies. Bull Mar Sci, 2001, 69(2): 579–593
Yu K F, Jiang M X, Chen Z Q, et al. Latest forty two years’ sea surface temperature change of Weizhou Island and its influence on coral reef ecosystem. Chin J Appl Ecol (in Chinese), 2004, 15(3): 506–510
Stimson J, Sakai K, Sembali H. Interspecific comparison of the symbiotic relationship in corals with high and low rates of bleaching-induced mortality. Coral Reefs, 2002, 21(4): 409–421
McClanahan T R, Baird A H, Marshall P A, et al. Comparing bleaching and mortality responses of hard corals between southern Kenya and the Great Barrier Reef, Australia. Mar Pollut Bull, 2004, 48(3–4): 327–335
Loya Y, Sakai K, Yamazato K, et al. Coral bleaching: the winners and the losers. Ecol Lett, 2001, 4: 122–131
Grimsditch G D, Salm R V. Coral Reef Resilience and Resistance to Bleaching. Gland: IUCN, 2006. 1–52
Wells J W. Scleractinia. In: Moore R C, ed. Treatise on Invertebrate Paleontology. Part F, Coelenterata. Kansas: The University of Kansas Press, 1956. 328–444
Chen C L, Yang Y W. Symbiont diversity in scleractinian corals from tropical reefs and subtropical non-reef communities in Taiwan. Coral Reefs, 2005, 24: 11–22
Toller W W, Rown R, Knowlton N. Zooxanthellae of the Montastraea annularis species complex: patterns of distribution of four taxa of Symbiodinium of different reefs and across depths. Biol Bull, 2001, 201: 348–359
Baker A C. Reef corals bleach to survive change. Nature, 2001, 411: 765–766
Fitt W K, McFarland F K, Warner M E, et al. Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef corals and relation to coral bleaching. Limnol Oceanogr, 2000, 45(3): 677–685
Shenkar N, Fine M, Kramarsky-Winter E, et al. Population dynamics of zooxanthellae during a bacterial bleaching event. Coral Reefs, 2006, 25: 223–227
Zhang Q M. On biogeomorphology of Luhuitou fringing reef of Sanya City, Hainan Island, China. Chin Sci Bull, 2001, 46(Suppl): 97–102
Zhang Q M, Shi Q, Chen G, et al. Status monitoring and health assessment of Luhuitou fringing reef of Sanya, Hainan, China. Chin Sci Bull, 2006, 51(Suppl II): 81–88
Chen Tianran, Yu Kefu, Shi Qi, et al. Scleractinian coral communities in Daya Bay: Current distribution and status (in Chinese), Tropical Geography, in press
Johannes R E, Wiebe W J. Method for determination of coral tissue biomass and composition. Limnol Oceanogr, 1970, 15(5): 822–824
Fagoonee I, Wilson H B, Hassell M P, et al. The dynamics of zooxanthellae populations: A long-term study in the field. Science, 1999, 283: 843–845
Costa C F, Sassi R, Amaral F D. Annual cycle of symbiotic dinoflagellates from three species of scleractinian corals from coastal reefs of northeastern Brazil. Coral Reefs, 2005, 24(2): 191–193
Jones R J. Zooxanthellae loss as a bioassay for assessing stress in corals. Mar Ecol Prog Ser, 1997, 149: 163–171
Zhu B H, Wang G C, Huang B. Effects of temperature, hypoxia, ammonia and nitrate on the bleaching among three coral species. Chin Sci Bull, 2004, 49(18): 1923–1928
Glynn P W. Extensive ‘bleaching’ and death of reef corals on the Pacific coast of Panama. Environ Conserv, 1983, 10: 149–154
Edwards A J, Clark S, Zahir H, et al. Coral bleaching and mortality on artificial and natural reefs in Maldives in 1998, sea surface temperature anomalies and initial recovery. Mar Pollut Bull, 2001, 42(1): 7–15
Warner M E, Fitt W K, Schmidt G W. Damage to photosystem II in symbiotic dinoflagellates: A determinant of coral bleaching. Proc Natl Acad Sci USA, 1999, 96(14): 8007–8012
Banaszak A T, Rowan M P, Kuffner I. et al. Relationship between ultraviolet (UV) radiation and mycosporinelike amino acids (MAAs) in marine organisms. Bull Mar Sci, 1998, 63: 617–628
Lesser M P, Stochaj W R, Tapley D W, et al. Bleaching in coral reef anthozoans: effects of irradiance, ultraviolet radiation, and temperature on the activities of protective enzymes against active oxygen. Coral Reefs, 1990, 8(4): 225–232
Salih A, Larkum A, Cox G, et al. Fluorescent pigments in corals are photoprotective. Nature, 2000, 408: 850–853
Warner M E, Chilcoat G C, McFarland F K, et al. Seasonal fluctuations in the photosynthetic capacity of photosystem II in symbiotic dinoflagellates in the Caribbean reef-building coral Montastraea. Marine Biology, 2002, 141: 31–38
Costa C F, Sassi R, Amaral F D. Population density and photosynthetic pigment content in symbiotic dinoflagellates in the Brazilian scleractinian coral Montastrea cavernosa. Braz J Oceanogr, 2004, 52(2): 93–99
Shi Q, Zhao M X, Zhang Q M, et al. Growth variations of scleratinan corals at Luhuitou, Sanya, Hainan Island, and the impacts from human activities. Acta Ecol Sin (in Chinese), 2007, 27(8): 3316–3323
Zhao M X, YU K F, Zhang Q M, Shi Q. The evolution and its environmental significance of coral diversity on Luhuitou fringing reef, Sanya (in Chinese). Marine Environmental Science, in press
Wu M L, Wang Y S. Using chemometrics to evaluate anthropogenic effects in Daya Bay, China. Estuar Coast Shelf S, 2007. 72(4): 732–742
Lambo A L. Ormond R F. Continued post-bleaching decline and changed benthic community of a Kenya coral reef. Mar Pollut Bull, 2006, 52(12): 1617–1624
Harithsa S, Raghukumar C, Dalal S G. Stress response of two coral species in the Kavaratti atoll of the Lakshadweep Archipelago, India. Coral Reefs, 2005, 24(3): 463–474
Brown B E, Le Tissier M D, Bythell J C. Mechanisms of bleaching deduced from histological studies of reef corals sampled during a natural bleaching event. Mar Biol, 1995, 122(4): 655–663
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Knowledge Innovation Project of the Chinese Academy of Sciences (Grant No. KZCX2-YW-318), the National Natural Science Foundation of China (Grant No. 40572102), the Ministry of Science and Technology of China (Grant No. 2006BAB19B03), and the Sino-Australia Special Collaboration Funds (Grant Nos. 4061120030 and CH050099)
About this article
Cite this article
Li, S., Yu, K., Shi, Q. et al. Interspecies and spatial diversity in the symbiotic zooxanthellae density in corals from northern South China Sea and its relationship to coral reef bleaching. Chin. Sci. Bull. 53, 295–303 (2008). https://doi.org/10.1007/s11434-007-0514-4
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11434-007-0514-4