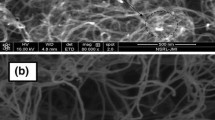
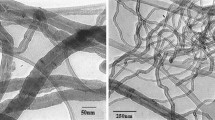
Abstract
In this paper, a new and efficient way to oxidize and functionalize the multi-walled carbon nanotubes (MWNTs) has been developed by using a combination of ultraviolet (UV) irradiation and Fenton oxidation process, namely UV/Fenton oxidation treatment. Comparing with conventionally individual Fenton oxidation treatment of MWNTs, UV/Fenton combined treatment improved the etching rates and efficiencies and hence reduced the time for surface modification of MWNTs, which was proved to be an effective method in etching and functionalizing CNTs. The formation of new functional groups, structural changes and thermal stability during oxidation period were characterized by Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy and could be clarified by thermogravimetric analysis (TGA), which showed that it was under UV irradiation conditions that MWNTs could be rapidly functionalized with hydroxyl, carbonyl and carboxyl groups in the presence of Fenton reagents, originating from the increase in the gross HO· concentration and the existent synergetic effect when using UV irradiation combing with Fenton oxidation process. Introduction of such new oxygen-containing functional groups was attributed to attacks of HO· on defect sites and unsaturated bonds of C=C in the MWNTs sample, which should play an important role in accounting for the FTIR and Raman spectral changes.
Similar content being viewed by others
References
Baughman R H, Zakhidov A A, De Heer W A, et al. Carbon nanotubes-the route toward applications. Science, 2002, 297(5582): 787–792
Bianco A, Kostarelos K, Partidos C D, et al. Biomedical applications of functionalized carbon nanotubes. Chem Commun, 2005, 5: 571–577
Bekyarova E, Ni Y, Malarkey E B, et al. Applications of carbon nanotubes in biotechnology and biomedicine. J Biomed Nanotechnol, 2005, 1(1): 3–17
Tang B Z, Xu H. Preparation, alignment, and optical properties of soluble poly(phenylacetylene)-wrapped carbon nanotubes. Macromolecules, 1999, 32(8): 2569–2576
Islam M F, Rojas E, Bergey D M, et al. High weight fraction surfactant solubilization of single-wall carbon nanotubes in water. Nano Lett, 2003, 3(2): 269–273
Moore V C, Strano M S, Haroz E H, et al. Individually suspended single-walled carbon nanotubes in various surfactants. Nano Lett, 2003, 3(10): 1379–1382
Petrov P, Stassin F, Pagnoulle C, et al. Noncovalent functionalization of multi-walled carbon nanotubes by pyrene containing polymers. Chem Commun, 2003, 23: 2904–2905
Shvartzman-Cohen R, Nativ-Roth E, Yerushalmi-Rozen R, et al. Selective dispersion of single-walled carbon nanotubes in the presence of polymers: the role of molecular and colloidal length scales. J Am Chem Soc, 2004, 126(45): 14850–14857
Sinani V A, Kotov N A, Yaroslavov A A, et al. Aqueous dispersions of single-wall and multi-wall carbon nanotubes with designed amphiphilic polycations. J Am Chem Soc, 2005, 127(10): 3463–3472
Tagmatarchis N, Prato M. Functionalization of carbon nanotubes via 1,3-dipolar cycloadditions. J Mater Chem, 2004, 14(4): 437–440
Hirsch A. Functionalization of single-walled carbon nanotubes. Angew Chem Int Ed, 2002, 41(11): 1853–1859
Georgakilas V, Kordatos K, Prato M, et al. Organic functionalization of carbon nanotubes. J Am Chem Soc, 2002, 124(5): 760–761
Pekke S, Salvetat J P, Jakab E, et al. Hydrogenation of carbon nanotubes and graphite in liquid ammonia. J Phys Chem B, 2001, 105(33): 7938–7943
Holzinger M, Vostrowsky O, Hirsch A, et al. Sidewall functionalization of carbon nanotubes. Angew Chem Int Ed, 2001, 40(21): 4002–4005
Rao C N R, Govindaraj A, Satishkumar B C, et al. Functionalized carbon nanotubes from solution, Chem Commun, 1996, 13: 1525–1526
Kuznetsova A, Popova I, Yates J T, et al. Oxygen-containing functional groups on singlewall carbon nanotubes: NEXAFS and vibrational spectroscopic studies. J Am Chem Soc, 2001, 123(43): 10699–10704
Yu Z, Brus L E. Reversible oxidation effect in Raman scattering from metallic single-wall carbon nanotubes. J Phys Chem A, 2000, 104(47): 10995–10999
Mawhinney D B, Naumenko V, Kuznetsova A, et al. Surface defect site density on single walled carbon nanotubes by titration. Chem Phys Lett, 2000, 324(1–3): 213–216
Cai L T, Bahr J L, Yao Y X, et al. Ozonation of single-walled carbon nanotubes and their assemblies on rigid self-assembled monolayers. Chem Mater, 2002, 14(10): 4235–4241
Pan H L, Liu L Q, Guo Z X. Carbon nanotubes from mechanochemical reaction. Nano Lett, 2003, 3(1): 29–32
Mickelson E T, Huffman C B, Rinzler A G. Fluorination of single wall carbon nanotubes. Chem Phys Lett, 1998, 296(1–2): 188–194
Kelly K F, Chiang I W, Mickelson E T. Insight into the mechanism of sidewall functionalization of single-walled nanotubes: an STM study Chem Phys Lett, 1999, 313(3–4): 445–450
Wu C D, Liu X H, Wang D B. Photosonochemical degradation of phenol in water. Wat Res, 2001, 35(16): 3927–3933
Shemer H, Kunukcu Y K, Linden K G. Degradation of the pharmaceutical metronidazole via UV, Fenton and photo-Fenton processes. Chemosphere, 2006, 63(2): 269–276
Gogate P R, Pandit A B. A review of imperative technologies for wastewater treatment II: hybrid methods. Adv Environ Res, 2004, 8(3–4): 553–597
Grujicic M, Cao G, Rao A M, et al. UV-light enhanced oxidation of carbon nanotubes. Appl Surf Sci, 2003, 214(1–4): 289–303
Valentini L, Armentano I, Kenny J M, et al. Interaction of oxygen with nanocomposites made of n-type conducting polymers and carbon nanotubes: role of charge transfer complex formation between nanotubes and poly(3-octylthiophene). Thin Solid Films, 2005, 476(1): 162–167
Savage T, Bhattacharya S, Sadanadan B, et al. Photoinduced oxidation of carbon nanotubes. J Phys: Condens Matter, 2003, 15(35): 5915–5921
Asano K, Kondo D, Kawabata A, et al. Chemical modification of multi-walled carbon nanotubes by vacuum ultraviolet irradiation dry process. Jpn J Appl Phys Part 1, 2006, 45(4B): 3573–3576
Simmons J M, Nichols B M, Baker, S E, et al. Effect of ozone oxidation on the single-walled carbon nanotubes. J Phys Chem B, 2006, 110(14): 7113–7118
Sham M L, Kim J K. Surface functionalities of multi-wall carbon nanotubes after UV/Ozone and TETA treatments. Carbon, 2006, 44(4): 768–777
Li W, Bai Y, Zhang Y K, et al. Effect of hydroxyl radical on the structure of multi-walled carbon nanotubes. Synth Met, 2005, 155(3): 509–515
Rodgers J D, Bunce N J. Treatment methods for the remediation of nitroaromatic explosives. Water Res, 2001, 35(9): 2101–2111
Arepalli S, Nikolaev P, Gorelik O. Protocol for the characterization of single-wall carbon nanotube material quality. Carbon, 2004, 42(8–9): 1783–1791
Chen X H, Chen C S, Chen Q, et al. Non-destructive purification of multi-walled carbon nanotubes produced by catalyzed CVD. Mat Lett, 2002, 57(3): 734–738
Chen C M, Chen M, Leu F C. Purification of multi-walled carbon nanotubes by microwave digestion method. Diam Relat Mater, 2004, 13(4–8): 1182–1186
Knight D S, White W B. Characterization of diamond films by Raman spectroscopy. J Mater Res, 1989, 4(2): 385–393
Kastner J, Pichler T, KuZmany H, et al. Resonance Raman and infrared spectroscopy of carbon nanotubes. Chem Phys Lett, 1994, 221(1–2): 53–58
Douglas B M, Naumenko V, Kuznetsova A Infrared spectral evidence for the etching of carbon nanotubes: Ozone oxidation at 298 K. J Am Chem Soc, 2000, 122(10): 2383–2384
Zhang J, Zou H L, Qing Q. Effect of chemical oxidation on the structure of single-walled carbon nanotubes. J Phys Chem B, 2003, 107(36): 3712–3718
Peng Y, Liu H W. Effect of oxidation by hydrogen peroxide on the structures of multi-walled carbon nanotubes. Ind Eng Chem Res, 2006, 45(19): 6483–6488
Tan P H, Zhang S L, Yue K T, et al. Comparative Raman study of carbon nanotubes prepared by D.C. Arc Discharge and catalytic methods. J Raman Spectrosc, 1997, 28(5): 369–372
Pimenta M A, Jorio A, Brown S D M. Diameter dependence of the Raman D-band in isolated single-wall carbon nanotubes. Phys Rev B, 2001, 64(4): 041401–041404
Itkis M E, Perea D E, Jung R, et al. Comparison of analytical techniques for purity evaluation of single-walled carbon nanotubes. J Am Chem Soc, 2005, 127(10): 3439–3448
Glaze W H, Kang J W. Advanced oxidation processes for treating groundwater contaminated with TCE and PCE: laboratory studies. J Am Water Works Assoc, 1988, 80(5): 57–63
Hong A, Zappi M E, Kuo C H, et al. Modeling the kinetics of illuminated and dark advanced oxidation processes. ASCE J Environ Eng, 1996, 122(1): 58–62
Cadot C, Dalko P I, Cossy J. Free-radical hydroxylation reactions of alkylboronates. J Org Chem, 2002, 67(21): 7193–7202
Vieira A J S C, Steenken S. Pattern of hydroxyl radical reaction with N6,N6,9-trimethyladenine: dehydroxylation and ring opening of isomeric hydroxyl adducts. J Phys Chem, 1991, 95(23): 9340–9346
Krauss M, Osman R. Electronic spectra of the neutral radical and H and OH adducts of uracil. J Phys Chem, 1993, 97(51): 13515
Sun T, Jia Z S, Xu Z D. Different hydroxyl radical scavenging activity of water-soluble β-alanine C[60] adducts. Bioorg Med Chem Lett, 2004, 14(7): 1779–1781
Zhang Z, Xiang Q, Glatt H, et al. Studies on pathways of ring opening of benzene in a Fenton system. Free Radic Biol Med, 1995, 18(3): 411–419
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by Shanghai Nanotechnology Promotion Center (Grant No. 0252nm011) and Doctoral R&D Fund of Henan Agricultural University (Grant No. 30200212)
About this article
Cite this article
Fan, C., Li, W., Li, X. et al. Efficient photo-assisted Fenton oxidation treatment of multi-walled carbon nanotubes. CHINESE SCI BULL 52, 2054–2062 (2007). https://doi.org/10.1007/s11434-007-0308-8
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11434-007-0308-8