Abstract
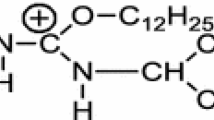
Density functional theory (DFT) of quantum chemistry was used to optimize the configuration of the anionic surfactant complexes CH3(CH2)7OSO −3 (H2O)n (n=0–6) and calculate their molecular frequencies at the B3LYP/6-311+G* level. The interaction of CH3(CH2)7OSO −3 with 1 to 6 water molecules was investigated at the air-water interface with DFT. The results revealed that the hydration shell was formed in the form of H-bond between the hydrophilic group of CH3(CH2)7OSO −3 and 6 waters. The strength of H-bonds belongs to medium. Binding free energy revealed that the hydration shell was stable. The increase of the number of water molecules will cause increases of the total charge of hydrophilic group and S10-O9-C8 bond angle, but decreases of the alkyl chain length and the bond lengths of S10-O11, S10-O12 as well as S10-O13, respectively.
Similar content being viewed by others
References
Zhao G X. Surfactant Physical Chemistry (revised) (in Chinese). Beijing: Peking University Press, 1991. 75
Gang C. Interaction decay of nonionic surfactants at water surfaces. Chem Phys Lett, 2003, 376(5): 758–760
Dominguez H, Berkowitz M L. Computer simulations of sodium Dodecyl sulfate at liquid/liquid and liquid/vapor interfaces. J Phys Chem B, 2000, 104(22): 5302–5308
Wei Y L, Rong Z M, Liu H L. Dynamic lattice Monte Carlo simulation of adsorption of Nonionic-surfactant s on oil-water interface. J Chem Ind Eng (in Chinese), 2005, 56(5): 894–899
Dong F L, Li Y, Zhang P. Mesoscopic simulation study on the orientation of surfactants adsorbed at the liquid/liquid interface. Chem Phys Lett, 2004, 399(1): 215–219
Ryszard Z, Henryk S. Structure of stable double-ionic model water clusters of quaternary alkyl ammonium surfactants with some monovalent counterions as derived by the DFT method. Int J Quant Chem, 2004, 99(5): 724–734
Yan X C, Luo D M, Zeng H, et al. Study on characters of electronic structures for anionic surfactants with different hydrophobic bases in gas and solvent using onsager model and ab initio method. Acta Chim Sin (in Chinese), 2004, 62(19): 1948–1950
Christian T, Daniel B A, Handy N C. Predicting the binding energies of H-bonded complexes: A comparative DFT study. Phys Chem Chem Phys, 1999, 1(17): 3939–3947
Alavi S, Thompson D L. H-bonding and proton transfer in small hydroxylammonium nitrate clusters: A theoretical study. J Chem Phys, 2003, 119(8): 4274
Cai Z L, Jeffrey R R. The first singlet (n,π*) and (π,π*) excited states of the hydrogen-bonded complex between water and pyridine. J Phys Chem A, 2002, 106(37): 8769–8778
Zhang B, Cai Y, Mu X L, et al. Multiphoton ionization and density functional studies of pyrimidine-(water)n clusters. J Chem Phys, 2002, 117(8): 3701–3710
Ahmed D, Ludwik A, Guido M. Density functional theory study of the hydrogen-bonded pyridine-H2O complex: A comparison with RHF and MP2 methods and with experimental data. J Phys Chem A, 2000, 104(10): 2112–2119
Oleg V S, Leonid G, Jerzy L. Does the hydrated cytosine molecule retain the canonical structure? A DFT Study. J Phys Chem B, 2000, 104(22): 5357–5361
Frisch M J, et al. GAUSSIAN 03, Revision C. 01, Gaussian, Inc., Wallingford, CT, 2004
Zhou G D, Duan L Y. The Base of Structural Chemistry (in Chinese). 3rd ed. Beijing: Peking University Press, 2003. 225
Rosen M J. Surfactants and Interfacial Phenomena. New York: Wiley, 1987. 210
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundations of China (Grant Nos. 20676051 and 20573048) and the Important Construction Project (Category A) of Shanghai Jiao Tong University (Grant No. AE150085)
About this article
Cite this article
Chen, M., Wang, Z., Wang, H. et al. Investigation of adsorption of surfactant at the air-water interface with quantum chemistry method. CHINESE SCI BULL 52, 1451–1455 (2007). https://doi.org/10.1007/s11434-007-0201-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11434-007-0201-5