Abstract
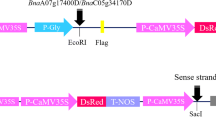
Integration of a transgene into a C-genome chromosome plays an important role in reducing ecological risk of transgenic Brassica napus. To obtain C-genome transgenic B. napus, herbicide-resistant bar gene was firstly transferred into B. oleracea var. alboglabra mediated by Agrobacterium tumefaciens strain LBA4404. Then using the transgenic B. oleracea as paternal plants and 8 non-transgenic varieties of B. rapa as maternal plants, C-genome transgenic B. napus with bar gene was artificially resynthesized by means of ovary culture and chromosome doubling. Among 67 lines of the resynthesized B. napus, 31 were positive, and 36 were negative according to PCR test for bar gene. At least 2 plants from each line were kept for PPT spray confirmation. The result was in consistence with the PCR test. Genomic Southern blotting of three randomly chosen lines also showed that bar gene had been integrated into the genome of resynthesized B. napus lines.
Similar content being viewed by others
References
Wilkinson M J, Elliott L J, Allainguillaume J, et al. Hybridization between Brassica napus and B. rapa on a national scale in the United Kingdon. Science, 2003, 302: 457–459
Mikkelsen T R, Jensen J, Jørgensen R B. Inheritance of oilseed rape (Brassica napus) RAPD markers in a backcross progeny with Brassica Campestris. Theor Appl Genet, 1996, 92: 492–497
Snow A A, Andersen B, Jørgensen R B. Costs of transgenic herbicide resistance introgressed from Brassica napus into weedy Brassica rapa. Mol Ecol, 1998, 8: 605–615
Hauser T P, Shaw R G, Østergård H. Fitness of F1 hybrids between weedy Brassica rapa and oilseed rape (B. napus). Heredity, 1998, 81: 429–435
Warkwick S I, Simard M J, Légère A, et al. Hybridization between transgenic Brassica napus L. and its wild relatives: B. rapa L., Raphanus raphanistrum L., Sinapis arvensis L., and Erucastrum gallicum (wild.) O. E. Schulz. Theor Appl Genet, 2003, 107: 528–539
Metz P L J, Nap J P. A transgene-centered approach to the biosafety of transgenic plants: Overview of selection and reporter genes. Acta Bot Neerl, 1997, 46: 25–50
Hauser T P, Jørgensen RB, østergárd H. Fitness of backcross and F2 hybrids between weedy Brassica rapa and oilseed rape (B. napus). Heredity, 1998, 81: 436–443
Metz P L J, Jacobsen E, Nap J P, et al. The impact on biosafety of the phosphinothricin-tolerance transgene in inter-specific B. rapa×B. napus hybrids and their successive backcrosses. Theor Appl Genet, 1997, 95: 442–450
Jørgensen R B, Andersen B, Hauser T P, et al. Introgression of crop genes from oilseed rape (Brassica napus) to related wild species: An avenue for the escape of engineered genes. Acta Horticulturae, 1998, 459: 211–217
Scheffler J A, Dale P J. Opportunities for gene transfer from transgenic oilseed rape (Brassica napus) to related species. Transgenic Res, 1994, 3: 263–278
Eastham K, Sweet J. Genetically modified organisms (GMOs): The significance of gene flow through pollen transfer. Environmental Issue Report No 28, Copenhagen: European Environment Agency, 2002. 75
Mikkelsen T R, Andersen B, Jørgensen R B. The risks of crop transgene spread. Nature, 1996, 380: 31
Zhu B, Lawrence J R, Warwick S I, et al. Inheritance of GFP-Bt transgenes from Brassica napus in backcrosses with three wild B.rapa accessions. Environ Biosafety Res, 2004, 3: 45–54
Lu C M, Kato M, Kakihara F. Destiny of a transgene escape from Brassica napus into Brassica rapa. Theor Appl Genet, 2002, 105: 78–84
Snowdon R J, Friedrich T, Friedt W, et al. Identifying the chromosomes of the A-and C-genome diploid Brassica species B. rapa (syn. campestris) and B. oleracea in their amphidiploid B. napus. Theor Appl Genet, 2002, 104: 533–538
Doyle J J, Doyle J I. Isolation of plant DNA from fresh tissue. Focus, 1990, 12: 13–15
Li Z, Liu H L, Luo P. Production and cytogeneric hybrids betwerassica napus and Orychophragmus violaceus. Theor Appl Genet, 1995, 91: 131–136
Sharpe A G, Parkin I A, Keith D J, et al. Frequent nonreciprocal translocations in the amphidiploid genome of oilseed rape (Brassica napus). Genome, 1995, 38: 1112–1121
Beversdorf W D, Weiss-Lerman J, Erickson Lr, et al. Transfer of cytoplasmically inherited triazine resistance from bird’s rape to cultivated oilseed rape (Brassica campestris and B. napus). Can J Genet Cytol, 1980, 22: 167–172
Jørgensen R B, Andersen B. Spontaneous hybridization between oilseed rape (Brassica napus) and weedy B. campestris (Brassicaceae): A risk of growing genetically modified oilseed rape. Am J Bot, 1994, 81: 1620–1626
Parkin I A P, Sharpe A G, Keith D J, et al. Identification of the A and C genomes of amphidiploid Brassica napus (oilseed rape). Genome, 1995, 38: 1122–1131
Parkin I A P, Lydiate D J. Conserved patterns of chromosome pairing and recombination in Brassica napus crosses. Genome, 1997, 40: 496–504
Namai H, Sarashima M, Hosoda T. Interspecific and inter-generic hybridization. In: Tsunoda S, Hinada K, Gomez-Campo C, eds. Brassica Crops and Wild Allies Biology and Breeding. Tokyo: Japanese Science Society Press, 1980. 191–202
Lu C M, Xiao L, Wu Y H. Ecological risk assessment of transgenic rapeseed in China. J Agricul Biotechnol, 2005, 13(3): 267–275
Nozaki T, Mishiba K, Mii M, et al. Construction of synteny groups of Brassica alboglabra by RAPD markers and detection of chromosome aberrations and distorted transmission under the genetic background of B. campestris. Theor Appl Genet, 2000, 101: 538–546
Lu C M. Fertilization fitness and relation to chromosome number in interspecific progeny between Brassica napus and B. rapa: A comparative study using natural and resynthesized B. napus. Breeding Science, 2001, 51: 73–81
Snow A A, Andersen B, Jørgensen R B. Costs of transgenic herbicide resistance introgressed from Brassica napus into weedy B. rapa. Mol Ecol, 1999, 8: 605–615
Tomiuk J, Hauser T P, Jørgensen R B. A-or C-chromosomes, does it matter for the transfer of transgenes from Brassica napus. Theor Appl Genet, 2000, 100: 750–754
U N. Genomic analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Japan J Bot, 1935, 7: 389–452
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Li, J., Fang, X., Wang, Z. et al. Transgene directionally integrated into C-genome of Brassica napus . CHINESE SCI BULL 51, 1578–1585 (2006). https://doi.org/10.1007/s11434-006-2021-4
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11434-006-2021-4