Abstract
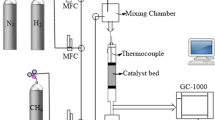
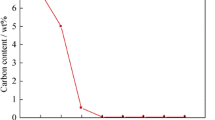
The splitting of carbon dioxide through the two-step solar thermochemical cycle presents enormous potential, for it holds the dual functionalities of solar fuel production and carbon-based energy recovery. However, the industrialization of this technology is impeded by two critical factors: The absence of fully developed oxygen carriers and advanced reaction devices that deliver exceptional performance. In order to identify a potentially effective oxygen carrier, 50 wt% NiO-modified CoFe2O4 is selected as the active component and characterized by means of thermogravimetry, scanning electron microscopy, and energy dispersive spectroscopy, so as to clarify its oxygen exchange capacity, micromorphology and elemental composition in high-temperature thermochemical cycles. Further, nanoparticle-coated foam-structured materials are prepared in combination with SiC ceramic foam for experimental testing in a high-flux solar reactor. The results indicate that a peak CO yield of 1.96 mL min−1 g−1 can be gained in a 1500–1250 K preliminary test, demonstrating the application potential of the material. In contrast to conventional redox materials, the CO2 activity of the materials synthesized in this study exhibits an enhancement with rising oxidation temperature. It means that isothermal cycles can potentially achieve higher conversion and fuel yield than non-isothermal cycles, while simultaneously reducing the amount of irreversible heat loss during high-temperature cycling. Although the estimated steady-state thermal efficiency of the solar reactor can reach up to 42%, further optimization of the reactor design is necessary to enhance energy conversion efficiency, as it is partially limited by the dimensions of the reaction chamber.
Similar content being viewed by others
References
Yan Y, Wang K, Clough P T, et al. Developments in calcium/chemical looping and metal oxide redox cycles for high-temperature thermochemical energy storage: A review. Fuel Process Tech, 2020, 199: 106280
Lu Y, Zhu L, Agrafiotis C, et al. Solar fuels production: Two-step thermochemical cycles with cerium-based oxides. Prog Energ Combust Sci, 2019, 75: 100785
Pullar R C, Novais R M, Caetano A P F, et al. A review of solar thermochemical CO2 splitting using ceria-based ceramics with designed morphologies and microstructures. Front Chem, 2019, 7
Bahari N A, Wan Isahak W N R, Masdar M S, et al. Clean hydrogen generation and storage strategies via CO2 utilization into chemicals and fuels: A review. Int J Energy Res, 2019, 43: 5128–5150
Chuayboon S, Abanades S. An overview of solar decarbonization processes, reacting oxide materials, and thermochemical reactors for hydrogen and syngas production. Int J Hydrogen Energy, 2020, 45: 25783–25810
Mehrpooya M, Habibi R. A review on hydrogen production thermochemical water-splitting cycles. J Cleaner Product, 2020, 275: 123836
Wittich K, Krämer M, Bottke N, et al. Catalytic dry reforming of methane: Insights from model systems. ChemCatChem, 2020, 12: 2130–2147
Haeussler A, Abanades S, Julbe A, et al. Solar thermochemical fuel production from H2O and CO2 splitting via two-step redox cycling of reticulated porous ceria structures integrated in a monolithic cavity-type reactor. Energy, 2020, 201: 117649
Mao Y, Gao Y, Dong W, et al. Hydrogen production via a two-step water splitting thermochemical cycle based on metal oxide—A review. Appl Energy, 2020, 267: 114860
Yadav D, Banerjee R. A review of solar thermochemical processes. Renew Sust Energy Rev, 2016, 54: 497–532
Riaz A, Ali M U, Enge T G, et al. Concentration-dependent solar thermochemical CO2/H2O splitting performance by Vanadia-Ceria multiphase metal oxide systems. Research, 2020, 2020: 3049534
Riaz A, Kreider P, Kremer F, et al. Electrospun manganese-based perovskites as efficient oxygen exchange redox materials for improved solar thermochemical CO2 splitting. ACS Appl Energy Mater, 2019, 2: 2494–2505
Wells V, Riaz A, Sun Q, et al. Mesoporous silica-encaged ultrafine ceria-nickel hydroxide nanocatalysts for solar thermochemical dry methane reforming. Appl Phys Lett, 2022, 120: 143905
Mustafa A, Lougou B G, Shuai Y, et al. Current technology development for CO2 utilization into solar fuels and chemicals: A review. J Energy Chem, 2020, 49: 96–123
Agrafiotis C, Roeb M, Sattler C. A review on solar thermal syngas production via redox pair-based water/carbon dioxide splitting thermochemical cycles. Renew Sust Energy Rev, 2015, 42: 254–285
Bayon A, Hashimoto J, Muhich C. Chapter Two—Fundamentals of solar thermochemical gas splitting materials. Adv Chem Eng, 2021, 58: 55–90
Villafán-Vidales H I, Arancibia-Bulnes C A, Riveros-Rosas D, et al. An overview of the solar thermochemical processes for hydrogen and syngas production: Reactors, and facilities. Renew Sust Energy Rev, 2017, 75: 894–908
Li D, Xu R, Li X, et al. Chemical looping conversion of gaseous and liquid fuels for chemical production: A review. Energy Fuels, 2020, 34: 5381–5413
Chuayboon S, Abanades S. Combined ZnO reduction and methane reforming for co-production of pure Zn and syngas in a prototype solar thermochemical reactor. Fuel Process Tech, 2021, 211: 106572
Fu M, Xu H, Ma H, et al. Theoretical assessment of hydrogen production and multicycle energy conversion via solar thermochemical cycle based on nonvolatile SnO2. J Energy Chem, 2019, 38: 177–184
Loutzenhiser P G, Meier A, Steinfeld A. Review of the two-step H2O/CO2-splitting solar thermochemical cycle based on Zn/ZnO redox reactions. Materials, 2010, 3: 4922–4938
Wang B, Li L, Pottas J J, et al. Thermal model of a solar thermochemical reactor for metal oxide reduction. J Sol Energy Eng, 2020, 142
Jin F, Xu C, Yu H, et al. CaCo0.05Mn0.95O3−δ: A promising perovskite solid solution for solar thermochemical energy storage. ACS Appl Mater Interfaces, 2021, 13: 3856–3866
Bayon A, de la Calle A, Ghose K K, et al. Experimental, computational and thermodynamic studies in perovskites metal oxides for thermochemical fuel production: A review. Int J Hydrogen Energy, 2020, 45: 12653–12679
Pan H, Li Y, Zhu L, et al. Solar-driven H2O/CO2 conversion to fuels via two-step electro-thermochemical cycle in a solid oxide electrochemical cell. Energy Convers Manage, 2022, 259: 115578
Shuai Y, Zhang H, Guene Lougou B, et al. Solar-driven thermochemical redox cycles of ZrO2 supported NiFe2O4 for CO2 reduction into chemical energy. Energy, 2021, 223: 120073
Li L, Yu H J, Li Y S, et al. Characteristics of the transient thermal load and deformation of the evacuated receiver in solar parabolic trough collector. Sci China Tech Sci, 2020, 63: 1188–1201
Geng B, Guene Lougou B, Shuai Y, et al. Design of gas-liquid two-phase separation device with application in solar hydrogen production system. Renew Sust Energy Rev, 2023, 175: 113169
Purohit S, Brooks G A. Chapter Five—Application of solar thermal energy to metallurgical processes. Adv Chem Eng, 2021, 58: 197–246
Bader R, Gampp L, Breuillé T, et al. Unsteady radiative heat transfer model of a ceria particle suspension undergoing solar thermochemical reduction. J Thermophys Heat Transfer, 2019, 33: 63–77
Almendros-Ibáñez J A, Fernández-Torrijos M, Díaz-Heras M, et al. A review of solar thermal energy storage in beds of particles: Packed and fluidized beds. Sol Energy, 2019, 192: 193–237
Tregambi C, Troiano M, Montagnaro F, et al. Fluidized beds for concentrated solar thermal technologies—A review. Front Energy Res, 2021, 9
Dai S, Chang Z, Chang C, et al. Numerical study on the directly-irradiated vortex reactor for solar CO2 coal gasification. Sol Energy, 2019, 188: 573–585
Wang Y, Li Q, Xuan Y. Thermal and chemical reaction performance analyses of solar thermochemical volumetric receiver/reactor with nanofluid. Energy, 2019, 189: 116123
Pan R, Debenest G. Numerical investigation of a novel smoldering-driven reactor for plastic waste pyrolysis. Energy Convers Manage, 2022, 257: 115439
Kaneko H, Miura T, Fuse A, et al. Rotary-type solar reactor for solar hydrogen production with two-step water splitting process. Energy Fuels, 2007, 21: 2287–2293
Marxer D, Furler P, Takacs M, et al. Solar thermochemical splitting of CO2 into separate streams of CO and O2 with high selectivity, stability, conversion, and efficiency. Energy Environ Sci, 2017, 10: 1142–1149
Shi X, Wang F, Lougou B G, et al. Experimental and numerical study regarding the biomimetic bone porous structure to match energy and mass flow in a solar thermochemical reactor. J Energy Storage, 2022, 55: 105645
Wu L, Lougou B G, Jiang B, et al. Experimentally validated numerical model of multi-spectral bands radiative transport in solar receiver/reactor with photo-active porous absorber reacting media. Energy Convers Manage, 2023, 278: 116740
Luo X, Shi J, Zhao C, et al. The energy efficiency of interfacial solar desalination. Appl Energy, 2021, 302: 117581
Deng S, Li Y. Constructing region-specific porous flow field to enhance heat, mass and charge transports in proton exchange membrane fuel cells. Energy Convers Manage, 2022, 274: 116459
Bhosale R R, Takalkar G, Sutar P, et al. A decade of ceria based solar thermochemical H2O/CO2 splitting cycle. Int J Hydrogen Energy, 2019, 44: 34–60
Chueh W C, Falter C, Abbott M, et al. High-flux solar-driven thermochemical dissociation of CO2 and H2O using nonstoichiometric ceria. Science, 2010, 330: 1797–1801
Haeussler A, Abanades S, Jouannaux J, et al. Recent progress on ceria doping and shaping strategies for solar thermochemical water and CO2 splitting cycles. AIMS Mater Sci, 2019, 6: 657–684
Riaz A, Bodger C I, Chen J, et al. Redox performance of Ceria-Vanadia mixed-phase reticulated porous ceramics for solar thermochemical syngas production. Energy Fuels, 2021, 35: 16791–16798
Jiang B, Sun Q, Guene Lougou B, et al. Highly-selective CO2 conversion through single oxide CuO enhanced NiFe2O4 thermal catalytic activity. Sust Mater Technol, 2022, 32: e00441
Zhang H, Zhang X, Yang D, et al. Selection of iron-based oxygen carriers for two-step solar thermochemical splitting of carbon dioxide. Energy Convers Manage, 2023, 279: 116772
Burra K G, Gupta A K, Kerdsuwan S. Isothermal splitting of CO2 to CO using cobalt-ferrite redox looping. J Energy Resour Tech, 2021, 143
Wang L, Ma T, Dai S, et al. Experimental study on the high performance of Zr doped LaCoO3 for solar thermochemical CO production. Chem Eng J, 2020, 389: 124426
Sanders M D, Bergeson-Keller A M, Coker E N, et al. A thermogravimetric temperature-programmed thermal redox protocol for rapid screening of metal oxides for solar thermochemical hydrogen production. Front Energy Res, 2022, 10: 856943
Zhang H, Shuai Y, Lougou B G, et al. Effects of foam structure on thermochemical characteristics of porous-filled solar reactor. Energy, 2022, 239: 122219
Zhang H, Shuai Y, Lougou B G, et al. Effects of multilayer porous ceramics on thermochemical energy conversion and storage efficiency in solar dry reforming of methane reactor. Appl Energy, 2020, 265: 114799
Zhang H, Guene Lougou B, Pan R, et al. Analysis of thermal transport and fluid flow in high-temperature porous media solar thermochemical reactor. Sol Energy, 2018, 173: 814–824
Zhang H, Shuai Y, Guene Lougou B, et al. Thermal characteristics and thermal stress analysis of solar thermochemical reactor under high-flux concentrated solar irradiation. Sci China Tech Sci, 2020, 63: 1776–1786
Warren K J, Tran J T, Weimer A W. A thermochemical study of iron aluminate-based materials: A preferred class for isothermal water splitting. Energy Environ Sci, 2022, 15: 806–821
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the National Key Research and Development Program of China (Grant No. 2018YFA0702300), the National Natural Science Foundation of China (Grant No. 52106085), and the Heilongjiang Postdoctoral Fund (Grant No. LBH-Z22120).
Rights and permissions
About this article
Cite this article
Zhang, H., Zhang, X., Yang, D. et al. Application of CoFe2O4-NiO nanoparticle-coated foam-structured material in a high-flux solar thermochemical reactor. Sci. China Technol. Sci. 66, 3276–3286 (2023). https://doi.org/10.1007/s11431-023-2397-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-023-2397-7