Abstract
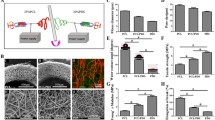
Mismatched biomechanical properties between artificial vascular grafts and native blood vessels can result in intimal hyperplasia, especially for the implantation of small-diameter blood vessels. Ideal biomaterials for vascular repair still remain challenged. Biodegradable poly(ε-caprolactone) (PCL) has been applied for the preparation of electrospun vascular grafts, but more efforts are needed to improve its compliance with tissue growth. Herein, L-arginine-based poly(ester urethane)urea (PEUU) with both elasticity and biodegradability was synthesized so as to enhance the biomechanical properties of vascular grafts by blending electrospinning with PCL in a given mass ratio. It was exhibited that the prepared electrospun PCL/PEUU fibrous membranes were suitable for cell proliferation with normal cell morphology. More importantly, the electrospun membrane with 1/1 mass ratio of PCL/PEUU (PEUU50) showed specific flexibility, exhibiting more suitable mechanical properties matching to the native blood vessels. Specifically, the PEUU50 electrospun membrane demonstrated significantly lower Young’s modulus (9.3±0.8 MPa) and tensile strength (6.0±0.5 MPa), and extreme higher elongation (389%±24%) in wet state than those (16.3±3.0 MPa, 11.4±7.1 MPa and 196%±57%, respectively) of the pristine PCL membrane. Overall, this study demonstrated the great potential of amino acid-based PEUUs for the application in small-diameter vascular grafts.
Similar content being viewed by others
References
Lopera Higuita M, Griffiths L G. Small diameter xenogeneic extracellular matrix scaffolds for vascular applications. Tissue Eng Part B-Rev, 2020, 26: 26–45
Gupta P, Mandal B B. Tissue-engineered vascular grafts: Emerging trends and technologies. Adv Funct Mater, 2021, 31: 2100027
Wolny R, Mintz G S, Pręgowski J, et al. Mechanisms, prevention and treatment of saphenous vein graft disease. Am J Cardiol, 2021, 154: 41–47
Leal B B J, Wakabayashi N, Oyama K, et al. Vascular tissue engineering: Polymers and methodologies for small caliber vascular grafts. Front Cardiovasc Med, 2020, 7: 592361
Li Z, Wu Z, Lu T, et al. Materials and surface modification for tissue engineered vascular scaffolds. J Biomater Sci Polym Ed, 2016, 27: 1534–1552
Hiob M A, She S, Muiznieks L D, et al. Biomaterials and modifications in the development of small-diameter vascular grafts. ACS Biomater Sci Eng, 2017, 3: 712–723
Li J, Cai Z, Cheng J, et al. Characterization of a heparinized decellularized scaffold and its effects on mechanical and structural properties. J Biomater Sci Polym Ed, 2020, 31: 999–1023
Bai S, Zhang X, Zang L, et al. Electrospinning of biomaterials for vascular regeneration. Chem Res Chin Univ, 2021, 37: 394–403
Fan S, Zhang Y, Huang X, et al. Silk materials for medical, electronic and optical applications. Sci China Technol Sci, 2019, 62: 903–918
Fang S, Ellman D G, Andersen D C. Review: Tissue engineering of small-diameter vascular grafts and their in vivo evaluation in large animals and humans. Cells, 2021, 10: 713
Wen M, Zhi D, Wang L, et al. Local delivery of dual microRNAs in trilayered electrospun grafts for vascular regeneration. ACS Appl Mater Interfaces, 2020, 12: 6863–6875
Wang Z, Zheng W, Wu Y, et al. Differences in the performance of PCL-based vascular grafts as abdominal aorta substitutes in healthy and diabetic rats. Biomater Sci, 2016, 4: 1485–1492
Zhu M, Wang Z, Zhang J, et al. Circumferentially aligned fibers guided functional neoartery regeneration in vivo. Biomaterials, 2015, 61: 85–94
Wu Y, Qin Y, Wang Z, et al. The regeneration of macro-porous electrospun poly(ε-caprolactone) vascular graft during long-term in situ implantation. J Biomed Mater Res B Appl Biomater, 2018, 106: 1618–1627
Obiweluozor F O, Emechebe G A, Kim D W, et al. Considerations in the development of small-diameter vascular graft as an alternative for bypass and reconstructive surgeries: A review. Cardiovasc Eng Tech, 2020, 11: 495–521
Zhu M, Wu Y, Li W, et al. Biodegradable and elastomeric vascular grafts enable vascular remodeling. Biomaterials, 2018, 183: 306–318
Woodard L N, Grunlan M A. Hydrolytic degradation and erosion of polyester biomaterials. ACS Macro Lett, 2018, 7: 976–982
van Uden S, Vanerio N, Catto V, et al. A novel hybrid silk-fibroin/polyurethane three-layered vascular graft: Towards in situ tissue-engineered vascular accesses for haemodialysis. Biomed Mater, 2019, 14: 025007
Yu E, Mi H, Zhang J, et al. Development of biomimetic thermoplastic polyurethane/fibroin small-diameter vascular grafts via a novel electrospinning approach. J Biomed Mater Res B Appl Biomater, 2018, 106: 985–996
Gostev A A, Chernonosova V S, Murashov I S, et al. Electrospun polyurethane-based vascular grafts: Physicochemical properties and functioning in vivo. Biomed Mater, 2020, 15: 015010
He M, Chu C C. A new family of functional biodegradable arginine-based polyester urea urethanes: Synthesis, chracterization and biodegradation. Polymer, 2013, 54: 4112–4125
Abel A K, Dreger N Z, Nettleton K, et al. Amino acid-based poly(ester urea)s as a matrix for extended release of entecavir. Biomacromolecules, 2020, 21: 946–954
Policastro G M, Lin F, Smith Callahan L A, et al. OGP functionalized phenylalanine-based poly(ester urea) for enhancing osteoinductive potential of human mesenchymal stem cells. Biomacromolecules, 2015, 16: 1358–1371
Zhu T, Gu H, Zhang H, et al. Covalent grafting of PEG and heparin improves biological performance of electrospun vascular grafts for carotid artery replacement. Acta Biomater, 2021, 119: 211–224
Zhu T, Yu K, Bhutto M A, et al. Synthesis of RGD-peptide modified poly(ester-urethane) urea electrospun nanofibers as a potential application for vascular tissue engineering. Chem Eng J, 2017, 315: 177–190
Yao Y, Ding J, Wang Z, et al. ROS-responsive polyurethane fibrous patches loaded with methylprednisolone (MP) for restoring structures and functions of infarcted myocardium in vivo. Biomaterials, 2020, 232: 119726
Du J, Wang J, Yu H, et al. Electrospun poly(p-dioxanone)/poly(esterurethane)ureas composite nanofibers for potential heart valve tissue reconstruction. Chin J Polym Sci, 2019, 37: 560–569
Zhu Q, Li X, Fan Z, et al. Biomimetic polyurethane/TiO2 nanocomposite scaffolds capable of promoting biomineralization and mesenchymal stem cell proliferation. Mater Sci Eng-C, 2018, 85: 79–87
Song H, Chu C C. Synthesis and characterization of a new family of cationic amino acid-based poly(ester amide)s and their biological properties. J Appl Polym Sci, 2012, 124: 3840–3853
You X, Gu Z, Huang J, et al. Arginine-based poly(ester amide) nanoparticle platform: From structure-property relationship to nucleic acid delivery. Acta Biomater, 2018, 74: 180–191
Kabirian F, Ditkowski B, Zamanian A, et al. Controlled NO-release from 3D-printed small-diameter vascular grafts prevents platelet activation and bacterial infectivity. ACS Biomater Sci Eng, 2019, 5: 2284–2296
He M, Potuck A, Kohn J C, et al. Self-assembled cationic biodegradable nanoparticles from pH-responsive amino-acid-based poly (ester urea urethane)s and their application as a drug delivery vehicle. Biomacromolecules, 2016, 17: 523–537
He M, Sun L, Fu X, et al. Biodegradable amino acid-based poly(ester amine) with tunable immunomodulating properties and their in vitro and in vivo wound healing studies in diabetic rats’ wounds. Acta Biomater, 2019, 84: 114–132
Yin M, Wan S, Ren X, et al. Development of inherently antibacterial, biodegradable, and biologically active chitosan/pseudo-protein hybrid hydrogels as biofunctional wound dressings. ACS Appl Mater Interfaces, 2021, 13: 14688–14699
Li Y, Zhang X, Ying B. On textile biomedical engineering. Sci China Technol Sci, 2019, 62: 945–957
Han J, Xiong L, Jiang X, et al. Bio-functional electrospun nanomaterials: From topology design to biological applications. Prog Polym Sci, 2019, 91: 1–28
Cui C, Wen M, Zhou F, et al. Target regulation of both VECs and VSMCs by dual-loading miRNA-126 and miRNA-145 in the bilayered electrospun membrane for small-diameter vascular regeneration. J Biomed Mater Res B Appl BioMater, 2019, 107: 371–382
Wen M, Zhou F, Cui C, et al. Performance of TMC-g-PEG-VAPG/miRNA-145 complexes in electrospun membranes for target-regulating vascular SMCs. Colloids Surfs B-Biointerfaces, 2019, 182: 110369
Zhang Q, Liu B, Chong J, et al. Combination of hydrophobically modified γ-poly(glutamic acid) and trehalose achieving high cryosurvival of RBCs. Sci China Technol Sci, 2021, 64: 806–816
Liu X, Gao S, Niu Q, et al. Facilitating trehalose entry into hRBCs at 4°C by alkylated ε-poly(L-lysine) for glycerol-free cryopreservation. J Mater Chem B, 2022, 10: 1042–1054
Braiman M S, Briercheck D M, Kriger K M. Modeling vibrational spectra of amino acid side chains in proteins: Effects of protonation state, counterion, and solvent on arginine C-N stretch frequencies. J Phys Chem B, 1999, 103: 4744–4750
Rickel A P, Deng X, Engebretson D, et al. Electrospun nanofiber scaffold for vascular tissue engineering. Mater Sci Eng-C, 2021, 129: 112373
Xie F, Zhang T, Bryant P, et al. Degradation and stabilization of polyurethane elastomers. Prog Polym Sci, 2019, 90: 211–268
Li T, Wang Y, Li S, et al. Mechanically robust, elastic, and healable ionogels for highly sensitive ultra-durable ionic skins. Adv Mater, 2020, 32: 2002706
Jeong Y J, Yao Y, Yim E K F. Current understanding of intimal hyperplasia and effect of compliance in synthetic small diameter vascular grafts. Biomater Sci, 2020, 8: 4383–4395
Stekelenburg M, Rutten M C M, Snoeckx L H E H, et al. Dynamic straining combined with fibrin gel cell seeding improves strength of tissue-engineered small-diameter vascular grafts. Tissue Eng Part A, 2009, 15: 1081–1089
Zheng Y, Ashizawa M, Zhang S, et al. Tuning the mechanical properties of a polymer semiconductor by modulating hydrogen bonding interactions. Chem Mater, 2020, 32: 5700–5714
Jirofti N, Mohebbi-Kalhori D, Samimi A, et al. Small-diameter vascular graft using co-electrospun composite PCL/PU nanofibers. Biomed Mater, 2018, 13: 055014
Oliveira S, Felizardo T, Amorim S, et al. Tubular fibrous scaffolds functionalized with tropoelastin as a small-diameter vascular graft. Biomacromolecules, 2020, 21: 3582–3595
Konig G, McAllister T N, Dusserre N, et al. Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery. Biomaterials, 2009, 30: 1542–1550
Jia W, Li M, Weng H, et al. Design and comprehensive assessment of a biomimetic tri-layer tubular scaffold via biodegradable polymers for vascular tissue engineering applications. Mater Sci Eng-C, 2020, 110: 110717
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the National Natural Science Foundation of China (Grant No. 52073204).
Supporting Information
The supporting information is available online at tech.scichina.com and link.springer.com. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Rights and permissions
About this article
Cite this article
Bai, S., Zhang, X., Zang, L. et al. Development of L-arginine-based poly(ester urethane)urea for enhanced vascular adaptability. Sci. China Technol. Sci. 65, 2751–2762 (2022). https://doi.org/10.1007/s11431-022-2038-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-022-2038-9