Abstract
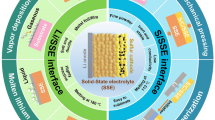
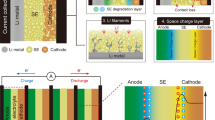
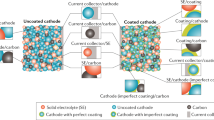
The development of lithium-ion solid electrolyte provides new ideas for solving the safety problems of secondary lithium-ion batteries (LIB) and, at the same time, the improvement of energy density. However, the consequent solid-solid interfacial problems have become a typical bottleneck to the performance. It has been considered that the space charge layer (SCL) formed at the interface to balance the sharp electrochemical inherent difference of properties is one of the most important factors that affect the ion transportation along and across the interface. Its existence may hinder ion transportation and increase the interfacial impedance but may also help to provide a new percolation path for lithium ion and so to enhance the ion migration. However the mechanism of SPL formation and its regulation on ion transport is not very clear, so it has raised a lot of attention and interest from LIB researchers. The purpose of this article is to try to gain some knowledge of SCL and, at the same time to browse through the related research in recent years. Hopefully, it may help those interested in solid electrolyte development for all-solid-state lithium-ion batteries (ASSB) in the future.
Similar content being viewed by others
References
Li S, Zhang S Q, Shen L, et al. Progress and perspective of ceramic/polymer composite solid electrolytes for lithium batteries. Adv Sci, 2020, 7: 1903088
Chai Y, Hasnain J, Bahl K, et al. Direct observation of nanoparticle-surfactant assembly and jamming at the water-oil interface. Sci Adv, 2020, 6: eabb8675
Wang H, An H, Shan H, et al. Research progress on interfaces of all-solid-state batteries. Acta Physico Chim Sin, 2020, 37: 2007070
Chen R, Li Q, Yu X, et al. Approaching practically accessible solidstate batteries: Stability issues related to solid electrolytes and interfaces. Chem Rev, 2020, 120: 6820–6877
Kato Y, Hori S, Saito T, et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat Energy, 2016, 1: 1–7
Long L, Wang S, Xiao M, et al. Polymer electrolytes for lithium polymer batteries. J Mater Chem A, 2016, 4: 10038–10069
Zheng F, Kotobuki M, Song S, et al. Review on solid electrolytes for all-solid-state lithium-ion batteries. J Power Sources, 2018, 389: 198–213
Li X, Ren Z, Norouzi Banis M, et al. Unravelling the chemistry and microstructure evolution of a cathodic interface in sulfide-based all-solid-state Li-ion batteries. ACS Energy Lett, 2019, 4: 2480–2488
Chen C, Ling S, Guo X, et al. Space charge layer effect in rechargeable solid state lithium batteries: Principle and perspective. Energy Storage Sci Tech, 2016, 5: 668–677
Singh M, Singh A K. Space charge layer induced superionic conduction and charge transport behaviour of “alkali carbonates and tri-doped ceria nanocomposites” for LT-SOFCs applications. Ceram Int, 2021, 47: 1218–1228
Wang L, Xie R, Chen B, et al. In-situ visualization of the spacecharge-layer effect on interfacial lithium-ion transport in all-solid-state batteries. Nat Commun, 2020, 11: 5889–5897
Katzenmeier L, Helmer S, Braxmeier S, et al. Properties of the space charge layers formed in Li-ion conducting glass ceramics. ACS Appl Mater Interfaces, 2021, 13: 5853–5860
Wang H, Zhu J, Su Y, et al. Interfacial compatibility issues in rechargeable solid-state lithium metal batteries: A review. Sci China Chem, 2021, 64: 879–898
Lehovec K. Space-charge layer and distribution of lattice defects at the surface of ionic crystals. J Chem Phys, 1953, 21: 1123–1128
Kliewer K L, Koehler J S. Space charge in ionic crystals. I. General approach with application to NaCl. Phys Rev, 1965, 140: A1226–A1240
Wagner C. The electrical conductivity of semi-conductors involving inclusions of another phase. J Phys Chem Solids, 1972, 33: 1051–1059
Jow T, Wagner J, Bruce J. The effect of dispersed alumina particles on the electrical conductivity of cuprous chloride. J Electrochem Soc, 1979, 126: 1963–1972
Maier J. Space charge regions in solid two-phase systems and their conduction contribution—I. Conductance enhancement in the system ionic conductor-‘inert’ phase and application on AgC1:Al2O3 and AgC1:SiO2. J Phys Chem Solids, 1985, 46: 309–320
Maier J. Space charge regions in solid two phase systems and their conduction contribution—II contact equilibrium at the interface of two ionic conductors and the related conductivity effect. Berichte Bunsengesellschaft Phys Chem, 1985, 89: 355–362
Chen C, Guo X. Space charge layer effect in solid state ion conductors and lithium batteries: Principle and perspective. Acta Chim Slov, 2016, 63: 489–495
Yamada H, Bhattacharyya A, Maier J. Extremely high silver ionic conductivity in composites of silver halide (AgBr, AgI) and mesoporous alumina. Adv Funct Mater, 2006, 16: 525–530
Duan H, Fan M, Chen W P, et al. Extended electrochemical window of solid electrolytes via heterogeneous multilayered structure for highvoltage lithium metal batteries. Adv Mater, 2019, 31: 1807789–1807795
Guo X, Waser R. Electrical properties of the grain boundaries of oxygen ion conductors: Acceptor-doped zirconia and ceria. Prog Mater Sci, 2006, 51: 151–210
Wu J F, Guo X. Origin of the low grain boundary conductivity in lithium ion conducting perovskites: Li3xLa0.67−xTiO3. Phys Chem Chem Phys, 2017, 19: 5880–5887
Badwal S, Ciacchi F T, Rajendran S, et al. An investigation of conductivity, microstructure and stability of electrolyte compositions in the system 9 mol% (Sc2O3-Y2O3)-ZrO2(Al2O3). Solid State Ion, 1998, 109: 167–186
Guo X, Maier J. Grain boundary blocking effect in zirconia: A schottky barrier analysis. J Electrochem Soc, 2001, 148: E121
Haruyama J, Sodeyama K, Han L, et al. Space-charge layer effect at interface between oxide cathode and sulfide electrolyte in all-solid-state lithium-ion battery. Chem Mater, 2014, 26: 4248–4255
de Klerk N J J, Wagemaker M. Space-charge layers in all-solid-state batteries; important or negligible? ACS Appl Energy Mater, 2018, 1: 5609–5618
Brogioli D, Langer F, Kun R, et al. Space-charge effects at the Li7 La3Zr2O12/poly(ethylene oxide) interface. ACS Appl Mater Interfaces, 2019, 11: 11999–12007
Usiskin R, Maier J. Interfacial effects in lithium and sodium batteries. Adv Energy Mater, 2021, 11: 2001455
Pervez S A, Kim G, Vinayan B P, et al. Overcoming the interfacial limitations imposed by the solid-solid interface in solid-state batteries using ionic liquid-based interlayers. Small, 2020, 16: 2000279–2000288
Li Y, Li Z. Research progress on the cathodic interfaces of all-soild-state lithium-ion batteries. Mater China, 2020, 39: 253–260
Yamada H, Suzuki K, Nishio K, et al. Interfacial phenomena between lithium ion conductors and cathodes. Solid State Ion, 2014, 262: 879–882
Tian H K, Jalem R, Gao B, et al. Electron and ion transfer across interfaces of the NASICON-type LATP solid electrolyte with electrodes in all-solid-state batteries: A density functional theory study via an explicit interface model. ACS Appl Mater Interfaces, 2020, 12: 54752–54762
Tsuchiya B, Ohnishi J, Sasaki Y, et al. In situ direct lithium distribution analysis around interfaces in an all-solid-state rechargeable lithium battery by combined ion-beam method. Adv Mater Interfaces, 2019, 6: 1900100
Gittleson F S, El Gabaly F. Non-faradaic Li+ migration and chemical coordination across solid-state battery interfaces. Nano Lett, 2017, 17: 6974–6982
Li S, Mohamed A I, Pande V, et al. Single-ion homopolymer electrolytes with high transference number prepared by click chemistry and photoinduced metal-free atom-transfer radical polymerization. ACS Energy Lett, 2018, 3: 20–27
Liu K, Li X, Cai J, et al. Design of high-voltage stable hybrid electrolyte with an ultrahigh Li transference number. ACS Energy Lett, 2021, 6: 1315–1323
Sakuda A, Hayashi A, Tatsumisago M. Sulfide solid electrolyte with favorable mechanical property for all-solid-state lithium battery. Sci Rep, 2013, 3: 2261
Li Y, Chen X, Dolocan A, et al. Garnet electrolyte with an ultralow interfacial resistance for Li-metal batteries. J Am Chem Soc, 2018, 140: 6448–6455
Duan H, Chen W P, Fan M, et al. Building an air stable and lithium deposition regulable garnet interface from moderate-temperature conversion chemistry. Angew Chem Int Ed, 2020, 59: 12069–12075
Ruan Y, Lu Y, Huang X, et al. Acid induced conversion towards a robust and lithiophilic interface for Li-Li7La3 Zr2 O12 solid-state batteries. J Mater Chem A, 2019, 7: 14565–14574
Takada K. Interfacial nanoarchitectonics for solid-state lithium batteries. Langmuir, 2013, 29: 7538–7541
Ohta N, Takada K, Zhang L, et al. Enhancement of the high-rate capability of solid-state lithium batteries by nanoscale interfacial modification. Adv Mater, 2006, 18: 2226–2229
Fingerle M, Buchheit R, Sicolo S, et al. Reaction and space charge layer formation at the LiCoO2-LiPON interface: Insights on defect formation and ion energy level alignment by a combined surface science-simulation approach. Chem Mater, 2017, 29: 7675–7685
Qiu Z, Zhang Y, Xia S, et al. Research progress on interface properties of inorganic solid state lithium ion batteries. Acta Chim Sin, 2015, 73: 992–1001
Ohta N, Takada K, Sakaguchi I, et al. LiNbO3-coated LiCoO2 as cathode material for all solid-state lithium secondary batteries. Electrochem Commun, 2007, 9: 1486–1490
Xu X, Takada K, Watanabe K, et al. Self-organized core-shell structure for high-power electrode in solid-state lithium batteries. Chem Mater, 2011, 23: 3798–3804
Zhao C Z, Zhang X Q, Cheng X B, et al. An anion-immobilized composite electrolyte for dendrite-free lithium metal anodes. Proc Natl Acad Sci USA, 2017, 114: 11069–11074
Liang J Y, Zeng X X, Zhang X D, et al. Mitigating interfacial potential drop of cathode-solid electrolyte via ionic conductor layer to enhance interface dynamics for solid batteries. J Am Chem Soc, 2018, 140: 6767–6770
Fan P, Liu H, Marosz V, et al. High performance composite polymer electrolytes for lithium-ion batteries. Adv Funct Mater, 2021, 31: 2101380
Zeng X X, Yin Y X, Shi Y, et al. Lithiation-derived repellent toward lithium anode safeguard in quasi-solid batteries. Chem, 2018, 4: 298–307
Liang J Y, Zeng X X, Zhang X D, et al. Engineering Janus interfaces of ceramic electrolyte via distinct functional polymers for stable highvoltage Li-metal batteries. J Am Chem Soc, 2019, 141: 9165–9169
Allcock H, Welna D, Maher A. Single ion conductors—Polyphosphazenes with sulfonimide functional groups. Solid State Ion, 2006, 177: 741–747
Jones S D, Nguyen H, Richardson P M, et al. Design of polymeric zwitterionic solid electrolytes with superionic lithium transport. ACS Cent Sci, 2022, 8: 169–175
Huo H, Li X, Sun Y, et al. Li2CO3 effects: New insights into polymer/garnet electrolytes for dendrite-free solid lithium batteries. Nano Energy, 2020, 73: 104836
Ling W, Fu N, Yue J, et al. A flexible solid electrolyte with multilayer structure for sodium metal batteries. Adv Energy Mater, 2020, 10: 1903966
Zhang Q, Liu K, Liu K, et al. Study of a composite solid electrolyte made from a new pyrrolidone-containing polymer and LLZTO. J Colloid Interface Sci, 2020, 580: 389–398
Zagórski J, López del Amo J M, Cordill M J, et al. Garnet-polymer composite electrolytes: New insights on local Li-ion dynamics and electrodeposition stability with Li metal anodes. ACS Appl Energy Mater, 2019, 2: 1734–1746
Connell J G, Fuchs T, Hartmann H, et al. Kinetic versus thermodynamic stability of LLZO in contact with lithium metal. Chem Mater, 2020, 32: 10207–10215
Jiang T, He P, Wang G, et al. Solvent-free synthesis of thin, flexible, nonflammable garnet-based composite solid electrolyte for all-solidstate lithium batteries. Adv Energy Mater, 2020, 10: 1903376–1903385
Liang C C. Conduction characteristics of the lithium iodide-aluminum oxide solid electrolytes. J Electrochem Soc, 1973, 120: 1289–1292
Maier J. Ionic conduction in space charge regions. Prog Solid State Chem, 1995, 23: 171–263
Hood Z D, Wang H, Li Y, et al. The “filler effect”: A study of solid oxide fillers with β-Li3PS4 for lithium conducting electrolytes. Solid State Ion, 2015, 283: 75–80
Huo H, Zhao N, Sun J, et al. Composite electrolytes of polyethylene oxides/garnets interfacially wetted by ionic liquid for room-temperature solid-state lithium battery. J Power Sources, 2017, 372: 1–7
Li Z, Huang H M, Zhu J K, et al. Ionic conduction in composite polymer electrolytes: Case of PEO:Ga-LLZO composites. ACS Appl Mater Interfaces, 2019, 11: 784–791
Zhang J, Zhao N, Zhang M, et al. Flexible and ion-conducting membrane electrolytes for solid-state lithium batteries: Dispersion of garnet nanoparticles in insulating polyethylene oxide. Nano Energy, 2016, 28: 447–454
Braun S, Yada C, Latz A. Thermodynamically consistent model for space-charge-layer formation in a solid electrolyte. J Phys Chem C, 2015, 119: 22281–22288
Zhao N, Mu S, Guo X X. Physical issues in solid garnet batteries. Acta Phys Sin, 2020, 69: 228804
Yamamoto K, Iriyama Y, Asaka T, et al. Dynamic visualization of the electric potential in an all-solid-state rechargeable lithium battery. Angew Chem Int Ed, 2010, 49: 4414–4417
Nomura Y, Yamamoto K, Hirayama T, et al. Direct observation of a Li-ionic space-charge layer formed at an electrode/solid-electrolyte interface. Angew Chem Int Ed, 2019, 58: 5292–5296
Xiang Y, Li X, Cheng Y, et al. Advanced characterization techniques for solid state lithium battery research. Mater Today, 2020, 36: 139–157
Aizawa Y, Yamamoto K, Sato T, et al. In situ electron holography of electric potentials inside a solid-state electrolyte: Effect of electricfield leakage. Ultramicroscopy, 2017, 178: 20–26
Zhang Y, Cao Z, Zheng F, et al. Measurement of space charge distribution for real long HV cable and uncertainties in space charge measurement. In: 2nd International Conference on Electrical Materials and Power Equipment (ICEMPE). Guangzhou, 2019. 31–33
Zhang J, Chi M, Chen Q, et al. Effect of temperature on space charge distribution in two layers of transformer oil and impregnated pressboard under DC voltage. IEEE Access, 2020, 8: 16647–16655
Cheng Z, Liu M, Ganapathy S, et al. Revealing the impact of spacecharge layers on the Li-ion transport in all-solid-state batteries. Joule, 2020, 4: 1311–1323
Shi S, Gao J, Liu Y, et al. Multi-scale computation methods: Their applications in lithium-ion battery research and development. Chin Phys B, 2016, 25: 018212
Yamamoto K, Yoshida R, Sato T, et al. Nano-scale simultaneous observation of Li-concentration profile and Ti-, O electronic structure changes in an all-solid-state Li-ion battery by spatially-resolved electron energy-loss spectroscopy. J Power Sources, 2014, 266: 414–421
Mier-Escurra G, Rodrigo-Mor A, Vaessen P. A calibration method for acoustic space charge measurements using multilayer samples. Sensors, 2018, 18: 2508–2518
Author information
Authors and Affiliations
Corresponding authors
Additional information
This work was supported by the Special Guiding Program for Technology Innovation of Shaanxi Province (Grant No. 2022GF05-02), the National Natural Science Foundation of China (Grant No. 21971117), the Nankai University Central University Function Research Fund (Grant No. 63186005), the Tianjin Rare Earth Key Laboratory of Materials and Application (Grant No. ZB19500202), the Open Fund of State Key Laboratory of Rare Earth Resources Utilization (Grant No. RERU2019001), the Project 111 (Grant No. B18030), the Collaborative Innovation Project of Beijing-Tianjin-Hebei (Grant No. 19YFSLQY00030), the National Key R&D Program of China (Grant No. 2021YFA1202400), the Outstanding Youth of Tianjin Natural Science Foundation Project (Grant No. 20JCJQJC00130), and the Key Project of Tianjin Natural Science Foundation (Grant No. 20JCZDJC00650).
Rights and permissions
About this article
Cite this article
Zhang, Q., Kong, Y., Gao, K. et al. Research progress on space charge layer effect in lithium-ion solid-state battery. Sci. China Technol. Sci. 65, 2246–2258 (2022). https://doi.org/10.1007/s11431-021-2027-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-021-2027-3