Abstract
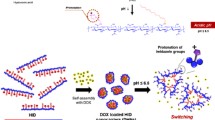
Disulfide (DSF) has been proved good anti-tumor effect and even better with coadministration of Cu2+. In this work, we report the use of hyaluronic acid (HA) based materials to construct vectors for the delivery of both DSF and Cu2+. HA was firstly modified with polyethylene glycol monomethyl ether (mPEG) and polycaprolactone (PCL) to synthesize an amphiphilic polymer (HA-PEG-PCL). DSF could be loaded in the hydrophobic core and Cu2+ could be cooperated to the negative hydrophilic segment. The Cu2+ also played a role as crosslinking agent, which prevented DSF leakage prematurely, avoiding the bad side effects to normal tissues. The interaction between HA and CD44 improved the distribution of nanodrugs in tumor cells. When the nanodrugs were delivered to the cancer cell, the acidic micro-environment would separate the Cu2+ from the surface, leading to the disintegration of the micelles, promoting the release of DSF from the micelle core. The results of in vitro and in vivo experiments showed that the DSF and Cu2+ co-delivery vector constructed in this work could enhance the antitumor effect and have low biological toxicity.
Similar content being viewed by others
References
Chen C, Sun W, Wang X, et al. pH-responsive nanoreservoirs based on hyaluronic acid end-capped mesoporous silica nanoparticles for targeted drug delivery. Int J Biol Macromolecules, 2018, 111: 1106–1115
Zhu C, Zhang H, Li W, et al. Suppress orthotopic colon cancer and its metastasis through exact targeting and highly selective drug release by a smart nanomicelle. Biomaterials, 2018, 161: 144–153
Feng H H, Pang L, Cong H L, et al. Preparation and application of fluorescence dendritic macromolecular nanoparticles. Integrated Ferroelectrics, 2019, 197: 99–110
Han X, Dong X, Li J, et al. Free paclitaxel-loaded E-selectin binding peptide modified micelle self-assembled from hyaluronic acid-paclitaxel conjugate inhibit breast cancer metastasis in a murine model. Int J Pharm, 2017, 528: 33–46
Zhong W, Pang L, Feng H, et al. Recent advantage of hyaluronic acid for anti-cancer application: A review of “3S” transition approach. Carbohydrate Polyms, 2020, 238: 116204
Kong L, Campbell F, Kros A. DePEGylation strategies to increase cancer nanomedicine efficacy. Nanoscale Horiz, 2019, 4: 378–387
Liu R, Xiao W, Hu C, et al. Theranostic size-reducible and no donor conjugated gold nanocluster fabricated hyaluronic acid nanoparticle with optimal size for combinational treatment of breast cancer and lung metastasis. J Control Release, 2018, 278: 127–139
Zhou L, Hu H, Zhang Y X, et al. A near-infrared triggered intracellular pH regulative PAMAM/O-nitrobenzaldehyde coated UCNPs for cancer therapy. Integrated Ferroelectrics, 2019, 199: 85–94
He B, Sui X, Wang S, et al. The stable ordered nanochannels based on block copolymer with acid-cleavable junction and UV crosslink group. Integrated Ferroelectrics, 2020, 206: 48–55
Liao J, Zheng H, Fei Z, et al. Tumor-targeting and pH-responsive nanoparticles from hyaluronic acid for the enhanced delivery of doxorubicin. Int J Biol Macromolecules, 2018, 113: 737–747
Liu Y, Qiao L, Zhang S, et al. Dual pH-responsive multifunctional nanoparticles for targeted treatment of breast cancer by combining immunotherapy and chemotherapy. Acta Biomater, 2018, 66: 310–324
Dong H, Pang L, Cong H, et al. Application and design of esterase-responsive nanoparticles for cancer therapy. Drug Deliver, 2019, 26: 416–432
Zhao Q, Liu J, Zhu W, et al. Dual-stimuli responsive hyaluronic acid-conjugated mesoporous silica for targeted delivery to CD44-over-expressing cancer cells. Acta Biomater, 2015, 23: 147–156
Saneja A, Nayak D, Srinivas M, et al. Development and mechanistic insight into enhanced cytotoxic potential of hyaluronic acid conjugated nanoparticles in CD44 overexpressing cancer cells. Eur J Pharmaceutical Sci, 2017, 97: 79–91
Nechushtan H, Hamamreh Y, Nidal S, et al. A phase IIb trial assessing the addition of disulfiram to chemotherapy for the treatment of metastatic non-small cell lung cancer. Oncologist, 2015, 20: 366–367
Skrott Z, Mistrik M, Andersen K K, et al. Alcohol-abuse drug disulfiram targets cancer via p97 segregase adaptor NPL4. Nature, 2017, 552: 194–199
Wu W, Yu L, Jiang Q, et al. Enhanced tumor-specific disulfiram chemotherapy by in situ Cu2+ chelation-initiated nontoxicity-to-toxicity transition. J Am Chem Soc, 2019, 141: 11531–11539
Liu P, Brown S, Goktug T, et al. Cytotoxic effect of disulfiram/copper on human glioblastoma cell lines and ALDH-positive cancer-stem-like cells. Br J Cancer, 2012, 107: 1488–1497
Brüning A, Kast R E. Oxidizing to death. Cell Cycle, 2014, 13: 1513–1514
Duan L, Shen H, Zhao G, et al. Inhibitory effect of disulfiram/copper complex on non-small cell lung cancer cells. Biochem Biophys Res Commun, 2014, 446: 1010–1016
Dosio F, Arpicco S, Stella B, et al. Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv Drug Deliver Rev, 2016, 97: 204–236
Hosseinzadeh H, Atyabi F, Varnamkhasti B S, et al. SN38 conjugated hyaluronic acid gold nanoparticles as a novel system against metastatic colon cancer cells. Int J Pharm, 2017, 526: 339–352
Nairi V, Magnolia S, Piludu M, et al. Mesoporous silica nanoparticles functionalized with hyaluronic acid. Effect of the biopolymer chain length on cell internalization. Colloids Surfs B-Biointerfaces, 2018, 168: 50–59
Liang X, Li X, Duan J, et al. Nanoparticles with CD44 targeting and ROS triggering properties as effective in vivo antigen delivery system. Mol Pharm, 2018, 15: 508–518
Lv Y, Xu C, Zhao X, et al. Nanoplatform assembled from a CD44-targeted prodrug and smart liposomes for dual targeting of tumor microenvironment and cancer cells. ACS Nano, 2018, 12: 1519–1536
Huang G, Huang H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliver, 2018, 25: 766–772
Gheran C V, Rigaux G, Callewaert M, et al. Biocompatibility of Gd-loaded chitosan-hyaluronic acid nanogels as contrast agents for magnetic resonance cancer imaging. Nanomaterials, 2018, 8: 201
Wang T, Yu X, Han L, et al. Tumor microenvironment dual-responsive core-shell nanoparticles with hyaluronic acid-shield for efficient co-delivery of doxorubicin and plasmid DNA. Int J Nanomedicine, 2017, Volume 12: 4773–4788
Lee J, Yoo K C, Ko J, et al. Hollow hyaluronic acid particles by competition between adhesive and cohesive properties of catechol for anticancer drug carrier. Carbohydrate Polyms, 2017, 164: 309–316
Gao Y, Hu L, Liu Y, et al. Targeted delivery of paclitaxel in liver cancer using hyaluronic acid functionalized mesoporous hollow alumina nanoparticles. Biomed Res Int, 2019, 2019: 1–10
Nguyen V D, Zheng S, Han J, et al. Nanohybrid magnetic liposome functionalized with hyaluronic acid for enhanced cellular uptake and near-infrared-triggered drug release. Colloids Surfs B-Biointerfaces, 2017, 154: 104–114
Wang H, Sun G, Zhang Z, et al. Transcription activator, hyaluronic acid and tocopheryl succinate multi-functionalized novel lipid carriers encapsulating etoposide for lymphoma therapy. Biomed Pharmaco Ther, 2017, 91: 241–250
Hong W G, Jeong G W, Nah J W. Evaluation of hyaluronic acid-combined ternary complexes for serum-resistant and targeted gene delivery system. Int J Biol Macromolecules, 2018, 115: 459–468
Li T, Yan L. Functional polymer nanocarriers for photodynamic therapy. Pharmaceuticals, 2018, 11: 133
Parmar M B, Meenakshi Sundaram D N, K.c. R B, et al. Combinational siRNA delivery using hyaluronic acid modified amphiphilic polyplexes against cell cycle and phosphatase proteins to inhibit growth and migration of triple-negative breast cancer cells. Acta Biomater, 2018, 66: 294–309
Giavaresi G, Torricelli P, Fornasari P M, et al. Blood vessel formation after soft-tissue implantation of hyaluronan-based hydrogel supplemented with copper ions. Biomaterials, 2005, 26: 3001–3008
Lan S, Wu X, Li L, et al. Synthesis and characterization of hyaluronic acid-supported magnetic microspheres for copper ions removal. Colloids Surfs A-Physicochem Eng Aspects, 2013, 425: 42–50
Cao X, Tao L, Wen S, et al. Hyaluronic acid-modified multiwalled carbon nanotubes for targeted delivery of doxorubicin into cancer cells. Carbohydrate Res, 2015, 405: 70–77
Zhao J, Wang Y, Ma Y, et al. Smart nanocarrier based on PEGylated hyaluronic acid for deacetyl mycoepoxydience: High stability with enhanced bioavailability and efficiency. Carbohydrate Polyms, 2019, 203: 356–368
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the National Natural Science Foundation of China (Grant Nos. 21675091, 21874078 and 22074072), the Taishan Young Scholar Program of Shandong Province (Grant No. tsqn20161027), the Natural Science Foundation of Shandong Province (Grant No. ZR2019BEM009), the Major Science and Technology Innovation Project of Shandong Province (Grant No. 2018CXGC1407), the Key Research and Development Project of Shandong Province (Grant Nos. 2016GGX102028, 2016GGX102039 and 2017GGX20111), the Innovation Leader Project of Qingdao (Grant No. 168325zhc), the Postdoctoral Scientific Research Foundation of Qingdao (Grant No. 40518060004), and the First Class Discipline Project of Shandong Province.
Supporting information
The supporting information is available online at tech.scichina.com and link.springerlink.com. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Pang, L., Zhong, W., Wang, Q. et al. Preparation and anti-tumor application of hyaluronic acid-based material for disulfide and copper ions co-delivery. Sci. China Technol. Sci. 64, 2023–2032 (2021). https://doi.org/10.1007/s11431-021-1841-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-021-1841-y