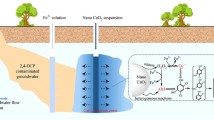
Abstract
An in situ groundwater remediation process, termed EGCW, was developed in this study by integrating in-well groundwater electrolysis into groundwater circulation well. Groundwater circulation carries electrolytically generated O2 and H2 into the impacted aquifer for in situ biodegradation of contaminants. In a two-dimensional tank filled with field sandy sediments, simulated trichloroethylene (TCE)-contaminated groundwater was circulated between an injection well with electrodes inside and a pumping well. Results from a 50-day EGCW experiment show that in-well electrolysis oxygenated most region near the injection well, and 10 mg/L TCE was mainly biodegraded aerobically to about 2.7 mg/L (73% removal) by the indigenous microbes. Aerobic TCE degradation was enhanced by the pulsed addition of acetate. Together with the proofs of stable carbon isotope fractionation (enrichment factor: −0.57‰–−1.53‰) and microbial community variation after EGCW treatment, aerobic cometabolism was proposed to be the most likely mechanism for TCE degradation. It is interesting to find that the intrinsic organic carbon in aquifer matrix could fuel the aerobic TCE degradation, particularly at low TCE concentrations. EGCW treatment is advantageous in terms of supplying appropriate dosages of electron acceptor (O2) and donor (H2) for in situ bioremediation because groundwater electrolysis and circulation are expedient and controllable.
Similar content being viewed by others
References
McCarty P L. Groundwater contamination by chlorinated solvents: History, remediation technologies and strategies. In: Stroo H F, Ward C H, eds. In Situ Remediation of Chlorinated Solvent Plumes. New York: Springer, 2010
Wan M, Liu R, Tang L R, et al. Pollution and statutes of volatile chlorinated hydrocarbons in soil and groundwater of industry (in Chinese). Environ Eng, 2011, 29: 397–411
Lu Q, Li H, Ling K F, et al. Investigation of chlorinated hydrocarbons in groundwater from a typical contaminated site in Pudong District, Shanghai (in Chinese). Acta Sci Circum, 2015, 36: 1730–1737
Lu H Y, Xin B D, Sun Y, et al. Temporal and spatial distribution characteristics of organic contamination in groundwater in the Beijing plain (in Chinese). Hydrogeol Eng Geol, 2014, 41: 34–40
Alleman B C. In-well treatment for chlorinated solvents remediation. In: Stroo H F, Ward C H, eds. In Situ Remediation of Chlorinated Solvent Plumes. New York: Springer, 2010
United States Environmental Protection Agency (USEPA). Field applications of in situ remediation technologies: Ground-water circulation wells. Technical Report. Washington DC: EPA, 1998. EPA 542-R-98-009, http://www.epa.gov/swertio1
Tawabini B, Makkawi M. Remediation of MTBE-contaminated groundwater by integrated circulation wells and advanced oxidation technologies. Water Sci Technol Water Supply, 2018, 18: 399–407
Dyer M, van Heiningen E, Gerritse J. A field trial for in-situ bior-emediation of 1,2-DCA. Eng Geol, 2003, 70: 315–320
Lakhwala F S, Mueller J G, Desrosiers R J. Demonstration of a microbiologically enhanced vertical ground water circulation well technology at a superfund site. Groundwater Monitor Remed, 1998, 18: 97–106
Němeček J, Steinová J, Špánek R, et al. Thermally enhanced in situ bioremediation of groundwater contaminated with chlorinated sol-vents—A field test. Sci Total Environ, 2018, 622–623: 743–755
Ponsin V, Coulomb B, Guelorget Y, et al. In situ biostimulation of petroleum hydrocarbon degradation by nitrate and phosphate injection using a dipole well configuration. J Contam Hydrol, 2014, 171: 22–31
Wu Q, Tu K, Sun H Z, et al. Investigation on the sustainability and efficiency of single-well circulation (SWC) groundwater heat pump systems. Renew Energy, 2019, 130: 656–666
Zhao Y S, Qu D, Zhou R, et al. Efficacy of forming biofilms by Pseudomonas migulae AN-1 toward in situ bioremediation of aniline-contaminated aquifer by groundwater circulation wells. Environ Sci Pollut Res, 2016, 23: 11568–11573
Geng Y K, Li Z H, Yuan L, et al. Degradation and mineralization of 2-chlorophenol in a single-stage anaerobic fixed-bed bioreactor. Sci China Tech Sci, 2020, 63: 86–95
Wang J, Reynolds D, Gent D. Electrokinetic-enhanced (EK-enhanced) amendment delivery for remediation of low permeability and heterogeneous materials. Technical Report. ESTCP Project ER-201325, 2018
Gilbert D M. Electrolytic reactive barriers forchlorinated solvent remediation. In: Stroo H F, Ward C H, eds. In Situ Remediation of Chlorinated Solvent Plumes. New York: Springer, 2010
EKOGRID. Five EKOGRIDTM Cases [OL]. Eko Harden Technologies Oy, 2019 [2019-12-26]. https://ekogrid.fi/wp/wp-content/uploads/2019/05/EKOGRID-Five-In-situ-Remediation-Cases.pdf
Goel R K, Liu L B, Flora J R V, et al. Modeling and comparison of electrolytic aeration and ORC® aeration of anoxic groundwater in a simulated laboratory aquifer. Environ Eng Sci, 2003, 20: 677–691
Franz J A, Williams R J, Flora J R V, et al. Electrolytic oxygen generation for subsurface delivery: Effects of precipitation at the cathode and an assessment of side reactions. Water Res, 2002, 36: 2243–2254
Jasmann J R, Gedalanga P B, Borch T, et al. Synergistic treatment of mixed 1,4-dioxane and chlorinated solvent contaminations by coupling electrochemical oxidation with aerobic biodegradation. Environ Sci Technol, 2017, 51: 12619–12629
Lohner S T, Tiehm A. Application of electrolysis to stimulate microbial reductive PCE dechlorination and oxidative VC biodegradation. Environ Sci Technol, 2009, 43: 7098–7104
Lohner S T, Becker D, Mangold K M, et al. Sequential reductive and oxidative biodegradation of chloroethenes stimulated in a coupled bioelectro-process. Environ Sci Technol, 2011, 45: 6491–6497
Weathers L J, Parkin G F, Alvarez P J. Utilization of cathodic hydrogen as electron donor for chloroform cometabolism by a mixed, methanogenic culture. Environ Sci Technol, 1997, 31: 880–885
Tiehm A, Lohner S T, Augenstein T. Effects of direct electric current and electrode reactions on vinyl chloride degrading microorganisms. Electrochim Acta, 2009, 54: 3453–3459
Wick L Y, Shi L, Harms H. Electro-bioremediation of hydrophobic organic soil-contaminants: A review of fundamental interactions. Electrochim Acta, 2007, 52: 3441–3448
Hunkeler D, Aravena R. Determination of compound-specific carbon isotope ratios of chlorinated methanes, ethanes, and ethenes in aqueous samples. Environ Sci Technol, 2000, 34: 2839–2844
Liu Y, Zhou A, Gan Y, et al. Variability in carbon isotope fractio-nation of trichloroethene during degradation by persulfate activated with zero-valent iron: Effects of inorganic anions. Sci Total Environ, 2016, 548–549: 1–5
Palau J, Soler A, Teixidor P, et al. Compound-specific carbon isotope analysis of volatile organic compounds in water using solid-phase microextraction. J Chromatogr A, 2007, 1163: 260–268
Darlington R, Lehmicke L, Andrachek R G, et al. Biotic and abiotic anaerobic transformations of trichloroethene and cis-1,2-di-chloroethene in fractured sandstone. Environ Sci Technol, 2008, 42: 4323–4330
Dong Y R, Liang X M, Krumholz L R, et al. The relative contributions of abiotic and microbial processes to the transformation of tetra-chloroethylene and trichloroethylene in anaerobic microcosms. Environ Sci Technol, 2009, 43: 690–697
Vancheeswaran S, Hyman M R, Semprini L. Anaerobic biotransformation of trichlorofluoroethene in groundwater microcosms. Environ Sci Technol, 1999, 33: 2040–2045
Zhang J J, Joslyn A P, Chiu P C. 1,1-dichloroethene as a predominant intermediate of microbial trichloroethene reduction. Environ Sci Technol, 2006, 40: 1830–1836
Frascari D, Zanaroli G, Danko A S. In situ aerobic cometabolism of chlorinated solvents: A review. J Hazard Mater, 2015, 283: 382–399
Shukla A K, Upadhyay S N, Dubey S K. Current trends in tri-chloroethylene biodegradation: A review. Critical Rev Biotech, 2014, 34: 101–114
Conrad M E, Brodie E L, Radtke C W, et al. Field evidence for co-metabolism of trichloroethene stimulated by addition of electron donor to groundwater. Environ Sci Technol, 2010, 44: 4697–4704
Gaza S, Schmidt K R, Weigold P, et al. Aerobic metabolic trichloroethene biodegradation under field-relevant conditions. Water Res, 2019, 151: 343–348
Schmidt K R, Gaza S, Voropaev A, et al. Aerobic biodegradation of trichloroethene without auxiliary substrates. Water Res, 2014, 59: 112–118
Jeannottat S, Hunkeler D. Chlorine and carbon isotopes fractionation during volatilization and diffusive transport of trichloroethene in the unsaturated zone. Environ Sci Technol, 2012, 46: 3169–3176
Chu K H, Mahendra S, Song D L, et al. Stable carbon isotope fractionation during aerobic biodegradation of chlorinated ethenes. Environ Sci Technol, 2004, 38: 3126–3130
Barth J A C, Slater G, Schüth C, et al. Carbon isotope fractionation during aerobic biodegradation of trichloroethene by Burkholderia cepacia G4: A tool to map degradation mechanisms. Appl Environ Microbiol, 2002, 68: 1728–1734
Clingenpeel S R, Moan J L, McGrath D M, et al. Stable carbon isotope fractionation in chlorinated ethene degradation by bacteria expressing three toluene oxygenases. Front Microbiol, 2012, 3: 63
Gafni A, Lihl C, Gelman F, et al. δ C and δ Cl isotope fractionation to characterize aerobic vs anaerobic degradation of trichloroethylene. Environ Sci Technol Lett, 2018, 5: 202–208
Nemir A, David M M, Perrussel R, et al. Comparative phylogenetic microarray analysis of microbial communities in TCE-contaminated soils. Chemosphere, 2010, 80: 600–607
Chen F, Li Z L, Liang B, et al. Electrostimulated bio-dechlorination of trichloroethene by potential regulation: Kinetics, microbial community structure and function. Chem Eng J, 2019, 357: 633–640
Ryoo D, Shim H, Canada K, et al. Aerobic degradation of tetrachloroethylene by toluene-o-xylene monooxygenase of Pseudomonas stutzeri OX1. Nat Biotechnol, 2000, 18: 775–778
Shim H, Ryoo D, Barbieri P, et al. Aerobic degradation of mixtures of tetrachloroethylene, trichloroethylene, dichloroethylenes, and vinyl chloride by toluene-o-xylene monooxygenase of Pseudomonas stutzeri OX1. Appl Microbiol Biotech, 2001, 56: 265–269
Liang S H, Liu J K, Lee K H, et al. Use of specific gene analysis to assess the effectiveness of surfactant-enhanced trichloroethylene co-metabolism. J Hazard Mater, 2011, 198: 323–330
Carere C R, McDonald B, Peach H A, et al. Hydrogen oxidation influences glycogen accumulation in a verrucomicrobial methanotroph. Front Microbiol, 2019, 10: 1873
Carere C R, Hards K, Houghton K M, et al. Mixotrophy drives niche expansion of verrucomicrobial methanotrophs. ISME J, 2017, 11: 2599–2610
Mohammadi S, Pol A, van Alen T A, et al. Methylacidiphilum fumariolicum SolV, a thermoacidophilic ‘Knallgas’ methanotroph with both an oxygen-sensitive and -insensitive hydrogenase. ISME J, 2017, 11: 945–958
Aulenta F, Reale P, Catervi A, et al. Kinetics of trichloroethene dechlorination and methane formation by a mixed anaerobic culture in a bio-electrochemical system. Electrochim Acta, 2008, 53: 5300–5305
Lampron K. Reductive dehalogenation of chlorinated ethenes with elemental iron: The role of microorganisms. Water Res, 2001, 35: 3077–3084
Yuan S H, Xie S W, Zhao K Y, et al. Field tests of in-well electrolysis removal of arsenic from high phosphate and iron groundwater. Sci Total Environ, 2018, 644: 1630–1640
Chu M Y J, Bennett P J, Dolan M E, et al. Concurrent treatment of 1,4-dioxane and chlorinated aliphatics in a groundwater recirculation system via aerobic cometabolism. Groundwater Monit R, 2018, 38: 53–64
Bagastyo AY, Radjenovic J, Mu Y, et al. Electrochemical oxidation of reverse osmosis concentrate on mixed metal oxide (MMO) titanium coated electrodes. Water Res, 2011, 45: 4951–4959
Gilbert D, Sale T, Petersen M. Electrically induced redox barriers for treatment of groundwater. Technical Report. ESTCP Project ER-0112, 2008
Mao X H, Yuan S H, Fallahpour N, et al. Electrochemically induced dual reactive barriers for transformation of TCE and mixture of contaminants in groundwater. Environ Sci Technol, 2012, 46: 12003–12011
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the National Key Research & Development Program of China (Grant No. 2018YFC1802504), the Natural Science Foundation of Hubei Province (Grant No. 2018CFA028), and the Fundamental Research Funds for the Central Universities, China University of Geosciences (Wuhan) (Grant No. CUGGC06).
Supplementary Information for
Rights and permissions
About this article
Cite this article
Yuan, S., Liu, Y., Zhang, P. et al. Electrolytic groundwater circulation well for trichloroethylene degradation in a simulated aquifer. Sci. China Technol. Sci. 64, 251–260 (2021). https://doi.org/10.1007/s11431-019-1521-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-019-1521-7