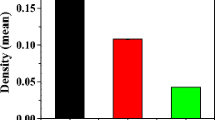
Abstract
Extracellular polymeric substances (EPS) extracted from electroactive bacteria show promising redox activity, but the electron transfer (ET) mechanism of the EPS has been rarely elucidated because of their structural complexity and lack of efficient methodologies. In this study, the charge transfer theory of surface-enhanced Raman spectroscopy (SERS) was applied to evaluate the redox properties of EPS adsorbed on Ni and Ag nanoparticles (NPs). These metal NPs were used to simultaneously magnify Raman signals and reduce/oxidize the redox moieties in EPS. As a result, the ET between EPS and metal NPs was evaluated through the changes in Raman signals. In this regard, we compared the redox activity of EPS extracted from two typical electroactive bacteria (Shewanella oneidensis and Geobacter sulfurreducens) and another two nonelectroactive strains (Escherichia coli and Bacillus subtilis). Electrochemical measurements show that the electroactive strains have higher redox capabilities than nonelectroactive strains. The SERS analysis shows that the porphyrin present in cytochrome c is the dominating redox moiety in the EPS of electroactive bacteria. The results of this study show that SERS with active metal substrates is a sensitive tool to probe the redox response of EPS, offering an opportunity to better understand the redox nature of biomolecules.
Similar content being viewed by others
References
Hernandez M E, Newman D K. Extracellular electron transfer. CMLS Cell Mol Life Sci, 2001, 58: 1562–1571
Logan B E. Exoelectrogenic bacteria that power microbial fuel cells. Nat Rev Micro, 2009, 7: 375–381
Jiang X, Chen Y, Hou C, et al. Promotion of para-chlorophenol reduction and extracellular electron transfer in an anaerobic system at the presence of iron-oxides. Front Microbiol, 2018, 9
Stams A J M, de Bok F A M, Plugge C M, et al. Exocellular electron transfer in anaerobic microbial communities. Environ Microbiol, 2010, 8: 371–382
Bae S, Joo J B, Lee W. Reductive dechlorination of carbon tetrachloride by bioreduction of nontronite. J Hazard Mater, 2017, 334: 104–111
Pant D, Singh A, Van Bogaert G, et al. Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. RSC Adv, 2012, 2: 1248–1263
Yuan Y, Chen Q, Zhou S, et al. Bioelectricity generation and microcystins removal in a blue-green algae powered microbial fuel cell. J Hazard Mater, 2011, 187: 591–595
Lovley D R. Bug juice: Harvesting electricity with microorganisms. Nat Rev Micro, 2006, 4: 497–508
Lovley D R. Electromicrobiology. Ann Rev Microbiol, 2012, 66: 391–409
El-Naggar M Y, Wanger G, Leung K M, et al. Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1. Proc Natl Acad Sci USA, 2010, 107: 18127–18131
Reguera G, McCarthy K D, Mehta T, et al. Extracellular electron transfer via microbial nanowires. Nature, 2005, 435: 1098–1101
Flemming H C, Wingender J, Szewzyk U, et al. Biofilms: An emergent form of bacterial life. Nat Rev Micro, 2016, 14: 563–575
Borole A P, Reguera G, Ringeisen B, et al. Electroactive biofilms: Current status and future research needs. Energy Environ Sci, 2011, 4: 4813–4834
Kang F, Qu X, Alvarez P J J, et al. Extracellular saccharide-mediated reduction of Au3+ to gold nanoparticles: New insights for heavy metals biomineralization on microbial surfaces. Environ Sci Technol, 2017, 51: 2776–2785
Xiao Y, Zhao F. Electrochemical roles of extracellular polymeric substances in biofilms. Curr Opin Electrochem, 2017, 4: 206–211
Wang J, Fu Z, Liu G, et al. Mediators-assisted reductive biotransformation of tetrabromobisphenol-A by Shewanella sp. XB. Bioresource Tech, 2013, 142: 192–197
Wang J, Lu H, Zhou Y, et al. Enhanced biotransformation of nitrobenzene by the synergies of Shewanella species and mediatorfunctionalized polyurethane foam. J Hazard Mater, 2013, 252–253: 227–232
Cao B, Ahmed B, Kennedy D W, et al. Contribution of extracellular polymeric substances from Shewanella sp. HRCR-1 biofilms to U(VI) immobilization. Environ Sci Technol, 2011, 45: 5483–5490
Cao B, Shi L, Brown R N, et al. Extracellular polymeric substances from Shewanella sp. HRCR-1 biofilms: Characterization by infrared spectroscopy and proteomics. Environ Microbiol, 2011, 13: 1018–1031
Li S W, Sheng G P, Cheng Y Y, et al. Redox properties of extracellular polymeric substances (EPS) from electroactive bacteria. Sci Rep, 2016, 6: 39098
Xiao Y, Zhang E, Zhang J, et al. Extracellular polymeric substances are transient media for microbial extracellular electron transfer. Sci Adv, 2017, 3: e1700623
Janissen R, Murillo D M, Niza B, et al. Spatiotemporal distribution of different extracellular polymeric substances and filamentation mediate Xylella fastidiosa adhesion and biofilm formation. Sci Rep, 2015, 5: 9856
Robuschi L, Tomba J P, Busalmen J P. Proving Geobacter biofilm connectivity with confocal Raman microscopy. J Electroanal Chem, 2016, 793: 99–103
Ly H K, Harnisch F, Hong S F, et al. Unraveling the interfacial electron transfer dynamics of electroactive microbial biofilms using surface-enhanced Raman spectroscopy. ChemSusChem, 2013, 6: 487–492
Li J, Cheng W, Wang X, et al. Electron transfer of cytochrome c on surface-enhanced raman scattering-active substrates: Material dependence and biocompatibility. Chem Eur J, 2017, 23: 9034–9038
Dai Y F, Xiao Y, Zhang E H, et al. Effective methods for extracting extracellular polymeric substances from Shewanella oneidensis MR-1. Water Sci Tech, 2016, 74: 2987–2996
Lee P C, Meisel D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J Phys Chem, 1982, 86: 3391–3395
Frølund B, Palmgren R, Keiding K, et al. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res, 1996, 30: 1749–1758
Fr/olund B, Griebe T, Nielsen P H. Enzymatic activity in the activated-sludge floc matrix. Appl Microbiol Biotechnol, 1995, 43: 755–761
Klüpfel L, Keiluweit M, Kleber M, et al. Redox properties of plant biomass-derived black carbon (Biochar). Environ Sci Technol, 2014, 48: 5601–5611
Sheng G P, Yu H Q, Li X Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotech Adv, 2010, 28: 882–894
Coble P G. Characterization of marine and terrestrial DOM in sea-water using excitation-emission matrix spectroscopy. Mar Chem, 1996, 51: 325–346
Zhu L, Qi H, Lv M, et al. Component analysis of extracellular polymeric substances (EPS) during aerobic sludge granulation using FTIR and 3D-EEM technologies. Bioresource Tech, 2012, 124: 455–459
Guo X, He L, Li Q, et al. Investigating the spatial variability of dissolved organic matter quantity and composition in Lake Wuliangsuhai. Ecol Eng, 2014, 62: 93–101
Lai C Y, Dong Q Y, Chen J X, et al. Role of Extracellular polymeric substances in a methane based membrane biofilm reactor reducing vanadate. Environ Sci Technol, 2018, 52: 10680–10688
Chen W, Westerhoff P, Leenheer J A, et al. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter. Environ Sci Technol, 2015, 37: 5701–5710
Ding P, Song W, Yang Z, et al. Influence of Zn(II) stress-induction on component variation and sorption performance of extracellular polymeric substances (EPS) from Bacillus vallismortis. Bioprocess Biosyst Eng, 2018, 41: 781–791
Chen J, Gu B, LeBoeuf E J, et al. Spectroscopic characterization of the structural and functional properties of natural organic matter fractions. Chemosphere, 2002, 48: 59–68
Shi L, Chen B, Wang Z, et al. Isolation of a high-affinity functional protein complex between OmcA and MtrC: Two outer membrane decaheme c-Type cytochromes of Shewanella oneidensis MR-1. J Bacteriology, 2006, 188: 4705–4714
Wang Z, Liu C, Wang X, et al. Kinetics of reduction of Fe(III) complexes by outer membrane cytochromes MtrC and OmcA of Shewanella oneidensis MR-1. Appl Environ MicroBiol, 2008, 74: 6746–6755
Rollefson J B, Stephen C S, Tien M, et al. Identification of an extracellular polysaccharide network essential for cytochrome anchoring and biofilm formation in Geobacter sulfurreducens. J Bacteriology, 2011, 193: 1023–1033
Lower B H, Yongsunthon R, Shi L, et al. Antibody recognition force microscopy shows that outer membrane cytochromes OmcA and MtrC are expressed on the exterior surface of Shewanella oneidensis MR-1. Appl Environ MicroBiol, 2009, 75: 2931–2935
Lovley D R, Fraga J L, Coates J D, et al. Humics as an electron donor for anaerobic respiration. Environ Microbiol, 1999, 1: 89–98
Newman D K, Kolter R. A role for excreted quinones in extracellular electron transfer. Nature, 2000, 405: 94–97
Lu Q, Chang M, Yu Z, et al. The effects of three commonly used extraction methods on the redox properties of extracellular polymeric substances from activated sludge. Environ Tech, 2015, 36: 2884–2891
Hu S, Morris I K, Singh J P, et al. Complete assignment of cytochrome c resonance Raman spectra via enzymic reconstitution with isotopically labeled hemes. J Am Chem Soc, 1993, 115: 12446–12458
Murgida D H, Hildebrandt P. Disentangling interfacial redox processes of proteins by SERR spectroscopy. Cheminform, 2008, 37: 937–945
Spiro T G, Strekas T C. Resonance Raman spectra of heme proteins. Effects of oxidation and spin state. J Am Chem Soc, 1974, 96: 338–345
Oellerich S, Wackerbarth H, Hildebrandt P. Spectroscopic characterization of nonnative conformational states of cytochrome c. J Phys Chem B, 2002, 106: 6566–6580
Lombardi J R, Birke R L. A unified view of surface-enhanced Raman scattering. Acc Chem Res, 2009, 42: 734–742
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 51678162, 41877045, 21876032), the Guangzhou City Science-Technology Project (Grant No. 201707010250), and the Major Scientific Project of Guangdong University (Grant No. 2017KZDXM029).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supporting Information
The supporting information is available online at tech.scichina.com and link.springer.com. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Electronic supplementary material
11431_2018_9437_MOESM1_ESM.doc
Molecular insight into electron transfer properties of extracellular polymeric substances of electroactive bacteria by surface-enhanced Raman spectroscopy
Rights and permissions
About this article
Cite this article
Tan, B., Zhou, S., Wang, Y. et al. Molecular insight into electron transfer properties of extracellular polymeric substances of electroactive bacteria by surface-enhanced Raman spectroscopy. Sci. China Technol. Sci. 62, 1679–1687 (2019). https://doi.org/10.1007/s11431-018-9437-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-018-9437-0