Abstract
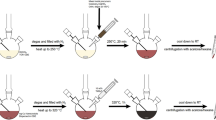
Ag@Cu2O core-shell metal-semiconductor nanoparticles (NPs) were prepared by using solution phase strategy. It was found that Ag@Cu2O core-shell NPs were easily converted to Ag@Cu bimetallic core-shell NPs with the help of surfactant PVP and excessive reducer ascorbic acid in air at room temperature, which is a unique phenomenon. Varying volumes of Ag colloidal solutions were added into the reaction mixtures containing fixed initial concentrations of Cu2+ and PVP, Ag@Cu2O and Ag@Cu core-shell NPs with fixed core size but varying outer shell thicknesses could be obtained. The composites, structures, morphologies and extinction properties of Ag@Cu2O and Ag@Cu core-shell NPs were systematically characterized by XRD, TEM and extinction spectra. Both of these NPs show wide tunable optical properties. The extinction peaks could be shifted from 421 nm to 700 nm. FTIR results reveal that Cu+ ions on the surface of Cu2O nanocrystalline coordinate with N and O atoms in PVP and further are reduced to metallic Cu by excessive ascorbic acid and then form a nucleation site on the surface of Cu2O nanocrystalline. PVP binds onto a different site to proceed with the reduction until all the Cu sources in Cu2O NPs are completely assumed. And the shell of Cu2O is converted to Cu shell. The synthesis approach in this paper is simple and also a promising reference for synthesizing other core-shell NPs. Ag@Cu2O NPs can be easily converted to Ag@Cu NPs in air at room temperature, which is promising to be used in electronic devices.
Similar content being viewed by others
References
Wang W C, Lyu L M, Huang M H. Investigation of the effects of polyhedral gold nanocrystal morphology and facets on the formation of Au-Cu2O core-shell heterostructures. Chem Mater, 2011, 23: 2677–2684
Kuo C H, Hua T E, Huang M H. Au nanocrystal-directed growth of Au-Cu2O core-shell heterostructures with precise morphological control. J Am Chem Soc, 2009, 131: 17871–17878
Liu D Y, Ding S Y, Lin H X, et al. Distinctive enhanced and tunable plasmon resonant absorption from controllable Au@Cu2O nanoparticles: experimental and theoretical modeling. J Phys C, 2012, 116: 4477–4483
Li J T, Cushing S K, Bright J, et al. Ag@Cu2O core-shell nanoparticles as visible-light plasmonic photocatalysts. ACS Catal, 2013, 3: 47–51
Yec C C, Zeng H C. Synthetic architecture of multiple core-shell and yolk-shell structures of (Cu2O@)n Cu2O (n=1–4) with centricity and eccentricity. Chem Mater, 2012, 24: 1917–1929
Kou J H, Saha A, Bennett-Stamper C, et al. Inside-out core-shell architecture: Controllable fabrication of Cu2O@Cu with high activity for the Sonogashira coupling reaction. Chem Commun, 2012, 48: 5862–5864
Li S K, Huang F Z, Wang Y, et al. Magnetic Fe3O4@C@Cu2O composites with bean-like core/shell nanostructures: Synthesis, properties and application in recyclable photocatalytic degradation of dye pollutants. J Mater Chem, 2011, 21: 7459–7466
Zhang L, Blom D A, Wang H. Au-Cu2O core-shell nanoparticles: A hybrid metal-semiconductor heteronanostructure with geometrically tunable optical properties. Chem Mater, 2011, 23: 4587–4598
Zhang L, Jing H, Boisvert G, et al. Geometry control and optical tunability of metal-cuprous oxide core-shell nanoparticles. ACS Nano, 2012, 6: 3514–3527
Jing H, Large N, Zhang Q F, et al. Epitaxial growth of Cu2O on Ag allows for fine control over particle geometries and optical properties of Ag-Cu2O core-shell nanoparticles. J Phys Chem C, 2014, 118: 19948–19963
Xiong J Y, Li Z, Chen J, et al. Facile synthesis of highly efficient one-dimensional plasmonic photocatalysts through Ag@Cu2O coreshell heteronanowires. ACS Appl Mater Interfaces, 2014, 6: 15716–15725
Kubota S, Morioka T, Takesue M, et al. Continuous supercritical hydrothermal synthesis of dispersible zero-valent copper nanoparticles for ink application in printed electronics. J Supercrit Fluid, 2014, 8: 33–40
Jang S, Seo Y, Choi J, et al. Sintering of inkjet printed copper nanoparticles for flexible electronics. Scripta Mater, 2010, 62: 258–261
Yand Q, Liang S H, Wang J, et al. Morphologicals and corrosion resistances of electroless Ni-P coated nanoporous coppers. Sci China Tech Sci, 2013, 56: 1147–1150
Zhao X H, Fuji M, Shirai T, et al. Electrocatalytic evolution of oxygen on NiCu particles modifying conductive alumina/nano-carbon network composite electrode. Sci China Tech Sci, 2012, 55: 3388–3394
Lee W R, Lim Y S, Kim S, et al. Crystal-to-crystal conversion of Cu2O nanoparticles to Cu crystals and applications in printed electronics. J Mater Chem, 2011, 21: 6928–6933
Liu D Q, Yang Z B, Wang P, et al. Preparation of 3D nanoporous copper-supported cuprous oxide for high-performance lithium ion battery anodes. Nanoscale, 2013, 5: 1917–1921
Paolella A, Brescia R, Prato M, et al. Colloidal synthesis of cuprite (Cu2O) octahedral nanocrystals and their electrochemical lithiation. ACS Appl Mater Interfaces, 2013, 5: 2745–2751
Chen L C. Review of preparation and optoelectronic characteristics of Cu2O-based solar cells with nanostructure. Mat Sci Semicon Proc, 2013, 16: 1172–1185
McShane C M, Choi K S. Junction studies on electrochemically fabricated p-n Cu2O homojunction solar cells for efficiency enhancement. Phys Chem Chem Phys, 2012,14: 6112–6118
Wang Z H, Zhao S P, Zhu A, et al. Photocatalytic synthesis of M/Cu2O (M=Ag, Au) heterogeneous nanocrystals and their photocatalytic properties. Cryst Eng Comm, 2011, 13: 2262–2267
Jiang T F, Xie T F, Chen L P, et al. Carrier concentration-dependent electron transfer in Cu2O/ZnO nanorod arrays and their photocatalytic performance. Nanoscale, 2013, 5: 2938–2944
Jana D, De G. Controlled and stepwise generation of Cu2O, Cu2O@Cu and Cu nanoparticles inside the transparent alumina films and their catalytic activity. RSC Adv, 2012, 2: 9606–9613
Palnichenko A V, Sidorov N S, Shakhrai D V, et al. Superconductivity of Cu/CuOx interface formed by shock-wave pressure. Physica C: Supercon, 2014, 498: 54–58
Singha R K. How the substitution of Zn for Cu destroys superconductivity in YBCO system? J Alloy Compd, 2010, 495: 1–6
Karamat S, Rawat R S, Tan T L, et al. Exciting dilute magnetic semiconductor: Copper-doped ZnO. J Supercon Nov Magn, 2013, 26: 187–195
Li B J, Cao H Q, Yin G, et al. Cu2O@reduced graphene oxide composite for removal of contaminants from water and supercapacitors. J Mater Chem, 2011, 21: 10645–10648
Hsu Y K, Yu C H, Chen Y C, et al. Hierarchical Cu2O photocathodes with nano/microspheres for solar hydrogen generation. RSC Adv, 2012, 2: 12455–12459
Meng F N, Di X P, Dong H W, et al. Ppb H2S gas sensing characteristics of Cu2O/CuO sub-microspheres at low-temperature. Sens Actuat B: Chem, 2013, 182:197–204
Andal V, Buvaneswari G. Preparation of Cu2O nano-colloid and its application as selective colorimetric sensor for Ag+ ion. Sens Actuat B, 2011, 155: 653–658
Zhuiykov S, Kats E, Marney D, et al. Improved antifouling resistance of electrochemical water quality sensors based on Cu2O-doped RuO2 sensing electrode. Prog Org Coat, 2011, 70: 67–73
Gao Z Y, Liu J L, Chang J L, et al. Mesocrystalline Cu2O hollow nanocubes: synthesis and application in non-enzymatic amperometric detection of hydrogen peroxide and glucose. Cryst Eng Comm, 2012, 14: 6639–6646
Sui Y M, Fu W Y, Yang H B, et al. Low temperature synthesis of Cu2O crystals: Shape evolution and growth mechanism. Cryst Growth Des, 2010, 10: 99–108
Wang D B, Mo M S, Yu D B, et al. Large-scale growth and shape evolution of Cu2O cubes. Cryst Growth Des, 2003, 3: 717–720
Paolella A, Brescia R, Prato M, et al. Colloidal synthesis of cuprite (Cu2O) octahedral nanocrystals and their electrochemical lithiation. ACS Appl Mater Interfaces, 2013, 5: 2745–2751
Yang A L, Wang Y J, Li S P, et al. Stepwise synthesis of cuprous oxide nanoparticles with adjustable structures and growth model. Sci China Tech Sci, 2014, 57: 2287–2294
Pang H, Gao F, Lu Q Y. Glycine-assisted double-solvothermal approach for various cuprous oxide structures with good catalytic activities. Cryst Eng Comm, 2010, 12: 406–412
Zhu H T, Wang J X, Xu G Y. Fast synthesis of Cu2O hollow microspheres and their application in DNA biosensor of hepatitis B virus. Cryst Growth Des, 2009, 9: 633–638
Liu G G, He F, Li X Q, et al. Three-dimensional cuprous oxide microtube lattices with high catalytic activity templated by bacterial cellulose nanofibers. J Mater Chem, 2011, 21: 10637–10640
Zhang L, Wang H. Cuprous oxide nanoshells with geometrically tunable optical properties. ACS Nano, 2011, 5: 3257–3267
Jiang T F, Xie T F, Yang W S, et al. Photoelectrochemical and photovoltaic properties of pn Cu2O homojunction films and their photocatalytic performance. J Phys Chem C, 2013, 117: 4619–4624
Zhang Z, Zhong C, Deng Y D, et al. The manufacture of porous cuprous oxide film with photocatalytic properties via an electrochemicalchemical combination method. RSC Adv, 2013, 3: 6763–6766
Pang H, Gao F, Lu Q Y. Glycine-assisted double-solvothermal approach for various cuprous oxide structures with good catalytic activities. Cryst Eng Comm, 2010, 12: 406–412
Haque E, Kim C M, Jhung S H. Facile synthesis of cuprous oxide using ultrasound, microwave and electric heating: Effect of heating methods on synthesis kinetics, morphology and yield. Cryst Eng Comm, 2011, 13: 4060–4068
Liu P S, Li Z G, Cai W P, et al. Fabrication of cuprous oxide nanoparticles by laser ablation in PVP aqueous solution. RSC Adv, 2011, 1: 847–851
Seoudi R, Fouda A A, Elmenshawy D A. Synthesis, characterization and vibrational spectroscopic studies of different particle size of gold nanoparticle capped with polyvinylpyrrolidone, Physica B, 2010, 405: 906–911
Xu Y Y, Chen D R, Jiao X L, et al. Nanosized Cu2O/PEG400 composite hollow spheres with mesoporous shells. J Phys Chem C, 2007, 111: 16284–16289
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, A., Li, S., Wang, Y. et al. Synthesis of Ag@Cu2O core-shell metal-semiconductor nanoparticles and conversion to Ag@Cu core-shell bimetallic nanoparticles. Sci. China Technol. Sci. 58, 881–888 (2015). https://doi.org/10.1007/s11431-015-5797-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-015-5797-0