Abstract
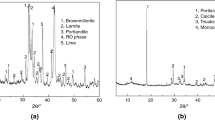
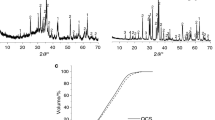
The influences of different alkaline conditions on the kinds and morphologies of steel slag’s hydration products, Ca(OH)2 content of hydration products, pore structure of hardened paste, non-evaporable water content of hydration products, and strength of steel slag mortar were investigated by changing the initial alkalinity of the hydration condition of steel slag. The results showed that increasing the initial alkalinity of hydration condition can promote the early hydration of steel slag’s active components (e.g., C2S, C3S, and C12A7), but it has little influence on their late-age hydration degree. The hydration degree of non-active components (e.g., RO phase and Fe3O4) of steel slag is very low even under strong alkaline condition with pH value of 13.8. The excitation effect of alkaline condition on the early hydration of steel slag is more obvious with the increase of pH value, but the kinds of steel slag’s hydration products are not influenced by changing the alkaline condition. The amount of steel slag’s hydration products is limited, so the strength of alkali-activated steel slag mortar is very low though the strong alkaline condition significantly promotes the early hydration of steel slag. Steel slag is not an ideal raw material for alkali-activated cementitious material.
Similar content being viewed by others
References
Aïtcin P C. Cements of yesterday and today: Concrete of tomorrow. Cem Concr Res, 2000, 30(9): 1349–1359
Mehta P K. Greening of the concrete industry for sustainable development. Concrete Int, 2002, (7): 23–28
Bentur A. Cementitious materials—mine millennia and a new century: Past, present, and future. J Mater Civ Eng, 2002, 14(1): 1–22
Mehta P K. Advances in concrete technology. Concr Int, 1999, 6: 69–76
Shi C J, Qian J S. High performance cementing materials from industrial slags—A review. Resour Conserv Recy, 2000, 29(3): 195–207
Berry E E, Malhotra V M. Fly ash for use in concrete—A critical review. ACI J, 1982, 2(3): 59–73
Mehta P K. Global concrete industry sustainability. Concr Int, 2009, 31(2): 45–48
Shi C J, Qian J S. Activated blended cement containing high volume coal fly ash. Adv Cem Res, 2001, 13(4): 157–163
Zhao F Q, Ni W, Wang H J, et al. Activated fly ash/slag blended cement. Resour Conserv Recy, 2007, 52: 303–313
Shi C J, Krivenko P V, Roy D M. Alkali-activated Cements and Concretes. London: Taylor & Francis Press, 2006
Li J X, Yu Q J, Wei J X, et al. Structural characteristics and hydration kinetics of modified steel slag. Cem Concr Res, 2011, 41(3): 324–329
Shi C J, Qian J S. High performance cementing materials from industrial slags-A review. Resour Conserv Recycl, 2000, 29(3): 195–207
Shi C J. Characteristics and cementitious properties of ladle slag fines from steel production. Cem Concr Res, 2002, 32: 459–462
Kourounis S, Tsivilis S, Tsakiridis P E, et al. Properties and hydration of blended cements with steelmaking slag. Cem Concr Res, 2007, 37: 815–822
Wang Q, Yan P Y. Hydration properties of basic oxygen furnace steel slag. Constr Build Mater, 2010, 24(7): 1134–1140
Deventer S J, Provis J. The Past, Present, and Future of Alkali-activated Binders. RILEM Technical Committee Report. In: International Conference on Advances in Construction Materials through Science and Engineering, Hong Kong SAR, China, 2011. 9
Wang Q, Yan P Y, Feng J W. A discussion on improving hydration activity of steel slag by altering its mineral compositions. J Hazard Mater, 2011, 186(2): 1070–1075
Hu S G, Wang H X, Zhang G Z, et al. Bonding and abrasion resistance of geopolymeric repair material made with steel slag. Cem Concr Comp, 2008, 30(3): 239–244
Sun J Y. Study of effects of ground steel slag on mechanical performance and soundness of concrete (in Chinese). Fly Ash, 2003, (5): 7–9
Wang B, Dai L P, Zhou M H. Experimental investigation on high performance concretewith fine grinded steel slag (in Chinese). J Shenyang Jianzhu Univ, 2003, 19(2): 148–149
Altun A, Yılmaz I. Study on steel furnace slags with high MgO as additive in Portland cement. Cem Concr Res, 2002, 32(8): 1247–1249
Rai A, Prabakar J, Raju C B, et al. Metallurgical slag as a component in blended cement. Constr Build Mater, 2002, 16(8): 489–494
Wang Q, Yan P Y, Han S. The influence of steel slag on the hydration of cement during the hydration process of complex binder. Sci China Tech Sci, 2011, 54: 1–6
Chen Y M, Zhang H T, Guo S H, et al. Study on the finely ground steel slag as high reactivity blending materials of cement (in Chinese). Cement, 2001, (5): 1–4
Zhong X L, Gong W L, Liang F Z. Properties and application of grinding slag being used as the additive of pumping concrete (in Chinese). Indust Constr, 1993, (7): 44–45
Lam L, Wong Y L, Poon C S. Degree of hydration and gel/space ratio of high-volume fly ash/cement systems. Cem Concr Res, 2000, 30(5): 747–756
Sarita R, Singh N B, Singh N P. Interaction of tartaric acid during hydration of Portland cement. Indian J Chem Tech, 2006, 13(5): 255–261
Bensted J, Barnes P. Structure and Performance of Cements. London: Taylor & Francis Press, 2002
Wu X Q, Zhu H, Hou X K, et al. Study on steel slag and fly ash composite Portland cement. Cem Concr Res, 1999, 29(7): 1103–1106
Tang M S, Yuan M X, Han S F, et al. The crystalline state of MgO, MnO, and FeO in steel slag and the soundness of steel slag cement (in Chinese). J Chin Ceram Soc, 1979, 7(1): 35–46
Xiao Q Z. Expansion and its inhibition of steel slag (in Chinese). J Chin Ceram Soc, 1996, 24(6): 635–640
Qian G R, Sun D D, Tay J H. Hydrothermal reaction and autoclave stability of Mg bearing RO phase in steel slag. British Ceramic Transs, 2002, 101(4): 159–164
Wachsmuth F, Geiseler J, Fix W, et al. Contribution to the structure of BOF-steel slags and its influence in their volume stability. C.I.M.M. In: International Symposium on Metallurgical Slags, Paper 5.8, Halifax, Canada. 1–18
Wang Q, Yan P Y, Han S. Activity index for steel slag. Mag Concr Res, 2011, 63(10): 737–742
Taylor H F W, Barret P, Brown P W, et al. The hydration of tricalcium silicate. Mater Struct, 1984, 17(6): 457–468
Jennings H M. Aqueous solubility relationships for two types of calcium silicate hydrate. J Am Ceram Soc, 1986, 69(8): 614–618
Mehta P K, Manmohan D. Pore size distribution and permeability of hardened cement paste. In: 7th International Symposium of the Chemistry of Cement, Paris, International Congress on the Chemistry of Cement, 1980. 1–5
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Q., Yang, J. & Yan, P. Influence of initial alkalinity on the hydration of steel slag. Sci. China Technol. Sci. 55, 3378–3387 (2012). https://doi.org/10.1007/s11431-012-4830-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-012-4830-9